Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Addition of hydroxylamine across double bonds
I am trying to prepare the reagent for tungsten described in this paper:
Attachment: Tungsten with Anti-1,5-di(p-methoxyphenyl-1-hydroxylamino-3-oximno-4- pentene JAnalChem Yoe & Jones 1944 p45-48.pdf (632kB)
This file has been downloaded 277 times
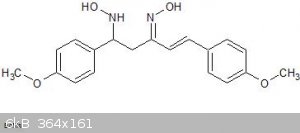
As you can see from the paper, they have not published the preparation but on examination of the structure it looks like it is the oxime of
di(4-methoxybenzal)-acetone with an extra hydroxylamine group added across one of the double bonds. I can imagine all sorts of complex routes to this
compound but my gut feeling is that it simply results from the treatment of a dibenzalacetone type compound with excess hydroxylamine.
Has anyone ever come across such a reaction before or have any ideas about this possible reaction and the conditions which might favor it?
Alternatively can anyone see an alternative route that looks realistic?
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Maybe the reaction is as simple as you speculate and goes automatically if hydroxylamine is in 2-fold molar excess to the enone.
I tried looking up the authors of the article, but nothing came up about this type of compound in general. I think it was just too valuable in those
days to publish the synthesis, they may kept it as a trade secret for themselves and tried to make some money from it.
Maybe a reaxys search on a simpler analog would be useful!
|
|
|
Cactuar
Harmless

Posts: 32
Registered: 25-7-2014
Location: Denmark
Member Is Offline
Mood: No Mood
|
|
Maybe one approach would be to condense 1 eq acetone with 1 eq anisaldehyde and then take the condensated product and condense that with 1 eq
anisaldehyde under milder conditions to get the beta-hydroxyketone. This could then by acylated. The acylated product would then react with an excess
of hydroxylamine which would form the product through both Sn2 of the acetoxy and condensation of the ketone.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Thanks guys. I will probably tackle waffles for a search but I will need to think about the nature of the search.
@ Cactaur, I like your idea and I can see some interesting possibilities for the second condensation. For example if the condensation occurs
under basic conditions could I use hydroxylamine as the base?
|
|
|
Cactuar
Harmless

Posts: 32
Registered: 25-7-2014
Location: Denmark
Member Is Offline
Mood: No Mood
|
|
That could be possible. Nitro-aldol condensations occur easily with amine bases. Maybe you could even do an acid condensation using hydroxylamine
hydrochloride, see attachment.
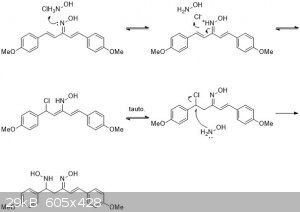
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I'd try this
https://en.wikipedia.org/wiki/Curcumin
Does anyone have access to this?
https://www.longdom.org/proceedings/transition-metal-complex...
According to the abstract "We provide a summarized synthesis and structural determination of Curcumin Oxime, Curcumin Th."
However, I have bad news; this might happen
https://pubs.rsc.org/en/content/articlelanding/1970/j3/j3970...
[Edited on 1-9-19 by unionised]
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Accidental find:
Harries, C.: Ann. [330] 185 (1903)
and
Trozzolo, A. M., and Lieber, E.: Anal. Chem. [22] 764 (1950)
The former reportedly speak about the addition of hydroxylamine to double bonds, the later is about the reaction with benzalacetophenone in alkaline
medium. (A series of reaction products.)
Maybe worth checking.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Pumukli, many thanks for the tip-off on these papers! I have read the Harries paper and it is exactly what I was looking for. I will have to read and
digest the relevant section again but this seem to comfirm my initial suspicions of a simple hydroxylamine addition across the double bond in
preference to oxime formation under the right conditons. I'll have a go with this reaction in the near future.
I haven't looked at the second paper yet (it took me most of the day to get through the first as its 95 pages long!!) but I am about to start.
I have some work to finish off on my nitro-chlorophenol sulphonic acid project before I move on to this one.
|
|
|