| Pages:
1
..
4
5
6
7
8
9 |
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Hopefully it is strong enough not to differentiate into another type of cell.  Sure looks that way though, nice welding job btw, MIG or TIG?.
Sure looks that way though, nice welding job btw, MIG or TIG?. 
Performing the synthesis at high temperature was also one of the things that I noticed to help get a more white product. From what I remember long
time ago, I used even higher temperatures with somewhat lower nitric concentrations, did you notice any difference in product there? I've also
wondered if it is really the same product as doublesalts formed at lower temperatures, as acetylene can also react with nitric under certain
conditions. Very interesting indeed, looking forward to the comparitative test!
[Edited on 8-2-2017 by nitro-genes]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
That is really welding and turning master-piece.
Absolutely far from amateur work...really professional.
Stem cells?...gigantic blast(o) cells I would rather say  ...with such a device
you can go up to the gram (1000 mg) range maybe even more if there is some venting and if the primary is not too powerfull. ...with such a device
you can go up to the gram (1000 mg) range maybe even more if there is some venting and if the primary is not too powerfull.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
markx
National Hazard
   
Posts: 645
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Quote: Originally posted by nitro-genes  | Hopefully it is strong enough not to differentiate into another type of cell.  Sure looks that way though, nice welding job btw, MIG or TIG?.
Sure looks that way though, nice welding job btw, MIG or TIG?. 
Performing the synthesis at high temperature was also one of the things that I noticed to help get a more white product. From what I remember long
time ago, I used even higher temperatures with somewhat lower nitric concentrations, did you notice any difference in product there? I've also
wondered if it is really the same product as doublesalts formed at lower temperatures, as acetylene can also react with nitric under certain
conditions. Very interesting indeed, looking forward to the comparitative test!
[Edited on 8-2-2017 by nitro-genes] |
Mitosis should not occur with this particular type of "stemcell"  
The welding was done with MIG and actually is quite sloppy and unpalatable even by my standard, but since I was going to trim off the rugged edges
then it makes little difference at the end.
As for going upwards of 65C I have noticed a quite distinct difference in the product properties already at around 70C.....it becomes more
coagulated/agglomerated and seems coarser. Also the potency seems to drop, have not measured quantitatively yet (it is in the plan though), but "match
shattering" results seem to indicate a downward spiral. I've gone as high as 90C and this resulted in a totally different product which was very
coarse and dense, yet lacked a lot of potency and seemed to perform much like the most dilute mix that I made with 50% KClO3. Also it
darkened considerably during damp storing in the dark (the fridge), something that never happens to 65C product even over a period of 12 months. I
think in the end I tossed the whole 90C batch as it seemed to posess a set of characteristics that I had no use for at the time.
Quote: Originally posted by PHILOU Zrealone  | That is really welding and turning master-piece.
Absolutely far from amateur work...really professional.
Stem cells?...gigantic blast(o) cells I would rather say  ...with such a device
you can go up to the gram (1000 mg) range maybe even more if there is some venting and if the primary is not too powerfull. ...with such a device
you can go up to the gram (1000 mg) range maybe even more if there is some venting and if the primary is not too powerfull. |
The gram range, I think, would be stretching it too far for this device....perhaps the chamber shall not subject to an act of multiple mitosis, but
the sheer volume of gas from such an amount of energetic will blow a lot of the sand out of the fuse channel and perhaps even take the brass cap off
along the way. I will happily drive this setup in the low hundreds of milligrams for high brisance secondaries without any fear of a possible mishap.
[Edited on 8-2-2017 by markx]
Exact science is a figment of imagination.......
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Welds look far better than my TIG ones for sure, so yeah... should remain a monoblast with ease! 
The almost linear dependence on temperature is interesting, maybe the amount of silver nitrate precipitated along with the carbide is sort of a
continiuum instead of some fixed possibilities of x-carbide-y-nitrate combinations? With silver being able to maybe also bind other ligands along the
way would be interesting to look at the precise composition of the stable product obtained.
[Edited on 8-2-2017 by nitro-genes]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1335
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
SADS
Markx method preparation was tested, all working perfectly. For dissolving 4g Ag was use 20g H2O + 28g HNO3/65. Use pure silver very fine powder and
process dissolving running only 30 second. In reactor by 66 C arises instantly white material after all time 10 min. Nothing problems with dirty
Istambul night CaC2.... I estimate - recommend big amount water in generator.
For example 500 - 1000ml. Maybe it working as partialy washing of gas. Only my opinion. Yield 4,8g SADS. Thanks Marks, Very scientific approach to
things..... I estimate - recommend big amount water in generator.
For example 500 - 1000ml. Maybe it working as partialy washing of gas. Only my opinion. Yield 4,8g SADS. Thanks Marks, Very scientific approach to
things..... ....LL ....LL
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
markx
National Hazard
   
Posts: 645
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Trying to make most of the Friday, I managed to shoot a series of samples in the "nr 36" cell to cover the dilution range of 0-40% KClO3 in
terms of limestone sand crushing ability.
The lot of 100mg samples:

My results of % dilution with KClO3 vs sand crushed below 100um in grams:

Due to a rather hectic schedule I only managed to shoot one series instead of four repetitions for every point. I will do that tomorrow to back up the
statistical aspect of the data, but since I carefully perpared all of the samples to have the same weight and also made sure the sand was homogenous
then even the single point results should reflect a reasonably credible picture of the situation...at least I hope so 
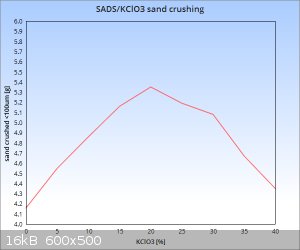
It seems that the sandstone from this shipment was a bit harder than the previous batch as the 100mg sample of pure SADS only crushed 4,16g below the
100 micron mark as opposed to 5,82g in my test with previous batch of sand. So already I have reached a situation where data from previous tests is
not directly comparable to current one. This makes it quite apparent that when comparable data is to be obtained, then working with materials of the
same batch throughout the whole series is absolutely mandatory. Luckily I have just enough of the sand stocked to complete this series with 4
repetition points for all dilution ratios and get a comparable set of data....also the SADS and KClO3 in all of the samples are from the same batch.
As for the relationship of brisance/sand crushing ability vs dilution ratio....seems that the sweet spot for this mix lingers with reasonable
probability somewhere between 20-25% KClO3 content. What is quite amazing is the fact that at 40% KClO3 content we are still at
about the level of pure double salt brisance or slightly higher (one datapoint is not enought to draw precise conclusions). Quite surprising, as I
suspected a sharper drop, but well, the preliminary data suggests otherwise....
Exact science is a figment of imagination.......
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Very nice markx,
You should plot your first results and the new ones in the same graph. Can you do it please?
As expected the geometric factor plays a role...
About the maximum ("sweet spot") actually it can be also between 15 and 20%...since 20% is your maximum graphical point from 5% intervals...the true
maximum can be right on it or on its right side or on its left side.
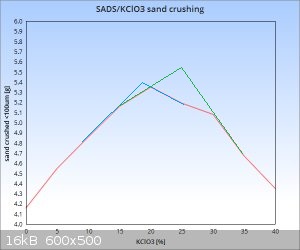
Also, once your process is mastered and reproductive...
I strongly invite you to make tinier intervals of 1%...
Once when working at Procter&Gamble, I was making a study of stiffeners to stop wrinkles of fabrics after laundry wash (post wash wrinkle
resistance project)...so we were screening silicons and activated them by ironing the fabrics...then the rigidity of the fabric was analysed by its
bending of a given lenght of rectangular calibrated piece of treated fabric.
My manager had chosen the % to analyse (1%, 3% ,5%, 10%)...normally you would expect an increase of rigidity of the fabric when increasing the amount
of rigidifier...but the results were erratic no matter the type of rigidifier...so I asked to my manager if I could perform an extensiver study, he
said OK but only during my free time...so I decided to perform a study on various product by 0.1% increments (thus instead of 4 point, I prepared,
tested and measured 100 solutions per product)...result was very unexpected...the rigidity was following an amorted oscillating patern and in fact the
use of 0.3% rigidifier was better than the use of 10%...so it had significant cost reduction impact.
[Edited on 11-2-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
markx
National Hazard
   
Posts: 645
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Quote: Originally posted by PHILOU Zrealone  | Very nice markx,
You should plot your first results and the new ones in the same graph. Can you do it please?
As expected the geometric factor plays a role...
About the maximum ("sweet spot") actually it can be also between 15 and 20%...since 20% is your maximum graphical point from 5% intervals...the true
maximum can be right on it or on its right side or on its left side.
Also, once your process is mastered and reproductive...
I strongly invite you to make tinier intervals of 1%...
Once when working at Procter&Gamble, I was making a study of stiffeners to stop wrinkles of fabrics after laundry wash (post wash wrinkle
resistance project)...so we were screening silicons and activated them by ironing the fabrics...then the rigidity of the fabric was analysed by its
bending of a given lenght or rectangular calibrated piece of treated fabric.
My manager had chosen the % to analyse (1%, 3% ,5%, 10%)...normally you would expect an increase of rigidity of the fabric when increasing the amount
of rigidifier...but the results were erratic no matter the type of rigidifier...so I asked to my manager if I could perform an extensiver study, he
said OK but only during my free time...so I decided to perform a study on various product by 0.1% increments (thus instead of 4 point, I prepared,
tested and measured 100 solutions per product)...result was very unexpected...the rigidity was following an amorted oscillating patern and in fact the
use of 0.3% rigidifier was better than the use of 10%...so it had significant cost reduction impact.
[Edited on 10-2-2017 by PHILOU Zrealone] |
Admirable persistence in executing a workload of an order of a magnitude greater than the initial plan and even more so outside of your required
duties....I do hope your employer rewarded your efforts with more than a subtle "thank you"  I myself have ventured on similar paths in service of similar employers in the past. Perhaps not so srikingly enourmous ones in terms of
workload, but found to my not so great surprise that besides satisfying personal curiosity for the topic, it gains one little leeway in the upward
direction of the rotten steps on the social ladder I myself have ventured on similar paths in service of similar employers in the past. Perhaps not so srikingly enourmous ones in terms of
workload, but found to my not so great surprise that besides satisfying personal curiosity for the topic, it gains one little leeway in the upward
direction of the rotten steps on the social ladder  But I am digressing from
the topic... But I am digressing from
the topic...
Sure I can and will include also the first results on a combined graph once I have completed the intended set of data. Perhaps displaying the results
in % of gain in the amount of sand crushed compared to the initial baseline of pure double salt shall display a clearer picture of the relationships
involved, as it offers a simple means to factor out the size of sample fired. The first results were obtained with 50mg energetic samples, but the
latter ones with 100mg amounts. Of course the "cell constant" may be different with dissimilar mass of charge, but the overall realtionship displayed
against the baseline in % difference between dilution ratios should be reasonably similar and comparable.
The "sweet point" proximity surely deserves a more enhanced resolution by smaller steps on the dilution range scale, but I would not venture on the
path of covering the whole scale with say a 1% resolution.....I highly doubt there are any sudden bursts of performance that might get lost between
the 5% resolution steps further away from the main peak area.
Exact science is a figment of imagination.......
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I have done the graph...
I have done the normalization... and the % increased performance graph too :-)
Was ready for posting two days ago, but the forum was unaccessible for me during two days (between 11/02 after midnight up to now) with a weird
message "Forbidden 403" and that I had no permission to acces it...this did also happened on 8/02 for a day long.
Here it is:
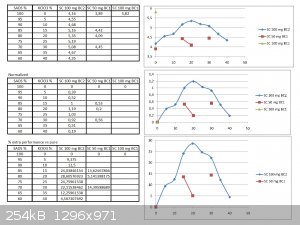
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
markx
National Hazard
   
Posts: 645
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
To continue the topic, below are my results from repetitive test of the same series under same conditions and with the same batches of materials:
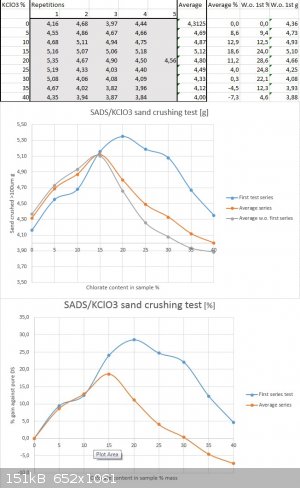
As one can without doubt see, there is noticeable and systematic dissonance between the first test series and the following ones regarding the range
past 15% chlorate content and upwards. The reason for it I have despite my sincerest efforts not discovered yet. But as it seems Philou was correct in
assuming that the maximum might be in either direction towards 15% as well as towards 20%. As the repetitive test shows it seems to be around 15%
chlorate content according to current results, despite my previous assumption that it might be more towards the 20% range. The devil lives in
repetitions, as one might say 
But to make things even more complicated I repeated the series of five datapoints per dilution ratio for both 15% and 20% chlorate content last
weekend to find out if the trendline is correct. To my great dissapointment I had again run out of testing medium and reused the sand that I had kept
from previous tests. To my surprise I received results that were below all of the previous ones in terms of sand crushing ability by as much as 1g
per datapoint for all shots fired. The trendline still revealed the maximum being near 15% though.
Either the previous testing knocked softer particles out of the testing medium and harder ones remained to show lesser brisance in retesting or the
double salt degraded during the week. The latter one is less probable and I think the dog is buried in the testing medium's altered properties. One
can not reuse it reliably and compare results (only if a full series is retested with the shot sand and calculated as % gain against a new setpoint).
Sigh....I ordered a new batch of filler with larger average particle size to be able to sieve out more of the useable 250/100um fraction.
I guess more testing around the "sweet spot" with the new fresh sand are on the way 
On a different note I also tried the "Stammzelle" function with 150 and 200mg ETN samples that were initiated by the 100mg SADS/KClO3 primary in
quartz sand medium. A satisfactory sharp "ping" note arose from the successful initiations and recoil launched the cell off the holder by about 15cm.
After sieve analysis it was concluded that 150mg sample had crushed 11,8g and 200mg sample 12,8g of quartz sand below the 100um mark. A satisfactory
result in terms of the cell function, but no more at the moment...
[Edited on 20-2-2017 by markx]
Exact science is a figment of imagination.......
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Very nice and surprizing...
To get a special view of the problem I have redone the graph...the normalization... and the % increased performance too :-)
From this one can see that results are not reproductible despite inside the same cell , with the same straws, fuses, geometry and the same batch of
SADS...this is obvious from the large distribution of the first points (pure SADS 100 mg) so the discrepancy must come from:
-the sand (and obviously his previous history)
-the pressure/compaction of the charge
-precision of the charge weighting (although if your scale is into the range of 1 mg...it must be 2% variation)
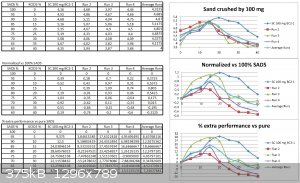
What is also stricking is the fact the "curve" is not a beautiful smooth Bell one but suffer from oscillating moves with shoulders...we may be into
the same kind of case scenario as the one I exposed earlier about my study at P&G 
I think that the first thing to do is to harmonize the sand batch...and to get more reliable results from pure SADS...
Maybe that the use of your special sand is not the best because not composed at 100% of silice...so the proportion of other softer ingredients may
vary (like CaCO3, clay micro-nuggets, ...) from batch to batch or even by simple segregation into the bag itself.
I will try to make a blastcell on my own...
Could you please give the dimensions of your two blast cells...external dimensions and internal chamber dimensions?
Since I don't have the same welding abilities as you and not the same material I will use plumbing pipes 4/4, 3/4 or 1/2...but I need to be close to
your system.
[Edited on 20-2-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
markx
National Hazard
   
Posts: 645
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Yes....the dissonance between the individual test results from experience this far has been much higher than I could anticipate in the beginning.
Exact reason for this remains a mystery and the nature of the drifting seems to be systematic througout the series. It is as if the scale would shift
every time I decide to shoot a series.....the first one was way up through the whole range...the second attempt of repetitions fell lower....and the
third attempt on 15% and 20% even more so.
The third one I can account for the recycled sand, but the shift between the first and second testing is unaccounted for as I really tried to keep the
conditions as close as I possibly could and the time between testing runs was only 24h, so any degradation of the energetic is not really possible on
that scale in this timeframe.
I do think that most of the wrinkles in the results are realted to the limestone sand and self seggregation of it in the storage vessel....plus the
altered average hardness of the used sand in third testing run.
The apparent occillations on the edges of the curve are perhaps more realted to the model of trendline approximation than the real nature of the
performance itself, although I dare not to oppose that position severly. Further testing will tell...
As for the cell dimensions I will give exact numbers tomorrow once I measure them properly, but the primary "36 cell" is very close to standard 1"
pipe in its main chamber, so using a threaded section and two end caps shall make a rather good copy of it for comparative testing.
As for using silica (quartz) sand for this material....probably not going to work out good. At least in my attempts the crushing action of sads
mixtures on silica sand was close to zero. Perhaps you may find a softer version, but the relative errors in measuring these small amounts of crushed
material may distort the results very significantly.
One option to enhance the crushing effect upon testing media and getting better resolution is to use bigger test samples of say 200-300mg each if one
does not mind the cost and has a plentiful supply of silver available.
All in all, despite the shifting between the series we still have a rather clear trendline through the dilution range, so I think something has been
revealed and the problems of data distortion can be fixed by correcting the testing procedure and parameters...I'm quite happy with the results and
the challenges that have been put in front of us 
Exact science is a figment of imagination.......
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
From Josef Köhler and Rudolf Meyer: Explosives , 4th edition
The "official" sand test usually use 400 mg pressed at 3000 psi into a N°6 cap and initiated by Pb(N3)2 or Hg(CNO)2 (and if needed by Pb(N3)2 with
Tetryl (200mg +250mg))...
The bomb contains 200g of 30 mesh Ottawa sand.
--> So this makes almost 1g explosive material in total (400+200+250=850 mg)
I have references of sand crushing tests with 600 mg and 1000mg...
I will put those datas here once put into graphics with all your new datas integrated.
From now on your results seems in agreement with other primaries...but I had to perform linear interpolation to get inexisting points...
Another possibility about the waves, is that somehow the geometry has an impact by a reverberating shockwave against the walls and a return crushing
wave.
This effect is observed with cylindrical lead block test (LBT) and only with certain explosives...thus generating weird symetrical cracks at specific
points of the LBT but generating bigger cavity than expected...to circumvent this they have made a system with hemispherical cylinder LBT (stil some
cracks but less and none close to the rounded part) and finally a full spherical LBT (crackless)...this proves that the geometry induced a
hydrodynamic process into the metallic lead caused by the shockwave...
[Edited on 21-2-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
markx
National Hazard
   
Posts: 645
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Excuse my fugly techical handdrawing....but that was the quickest and most effortless way of getting the dimensions visualised for making a close
replica:
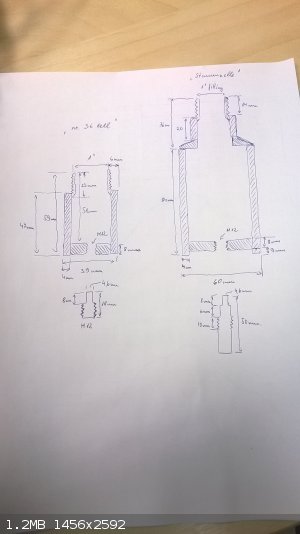
The smaller "36 cell" holds 40g of sand and "Stammzelle" exactly 200g....was not intending to copy literature value, this is pure coincidence. I must
say that this sample holder design is not really suitable for testing of high brisance secondaries and there needs to be some cm of clearance between
the sample holder and the actual high brisance sample. Even with the relatively "tiny" 150mg and 200mg ETN samples I can see that the sample holder
has been deformed and crushed in on itself. Measuring with caliper reveals that the top end of the sample stem has become 0,2mm wider than the bottom
only after 2 test shots with minimal energetic content. The holder has been fabricated from a rather strong 10,9 strength class bolt and it is tough
as a coffin nail, but no match to the direct pressure wave from ETN and with this rate of degradation it is not going to last even one testing series.
Exact science is a figment of imagination.......
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Thank you Markx, that is more than perfect...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
markx
National Hazard
   
Posts: 645
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Ok....more surprising nuances that I just discovered. I built an electrical ignition system for the "nr 36" cell, based on a small high voltage "tazer
like" module that creates an electrical discharge on the surface of SADS sample between two electrodes. And I mean a nasty bright, loud, fat arc
discharge that burns through paper with ease and chars it. Something that by the sound and looks of it will really make one feel the shock if it
should contact your system. Loaded it all into the cell under the sand and pushed the button......one can hear the arc from under the sand going at
about 50Hz discharge rate and NO INITIATION!! I let it crackle for about 5 seconds straight....and nada, nothing, no bump, no bang, no ping.
I dissassembled the cell and pulled the sample out, totally intact. Imagine that, the surface was scarred black and also the walls of the plastic
straw were covered in black deposit.....so there was no doubt that the arc touched the sample and decomposed the surface layer, but it did not ignite
the primary. So I sprinkled some loose SADS on top of the pressed sample and reassembled the system so that the electrode tips were immersed in the
loose acetylide. This time the sample went off straight away, so I repeated the test with another sample, but this time I added a bit less loose SADS
on top. Not a complete layer, but chunks around the electrodes with a bit free space inbetween them.....and got no ignition! Upon dissassembly I again
found that the surface was scorched and also the loose bits were dark colored, but no go nevertheless.
It seems that the much talked about sensitivity of this compound towards electrical discharge might be a bit overstated. Only if the discharge is
forced directly through the loose layer of SADS an ignition might occur.
  
Notice the black goop on the inside of the sample tube.....that was caused by the arc burning on the surface.
Exact science is a figment of imagination.......
|
|
|
MineMan
National Hazard
   
Posts: 998
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
I agree, SADS is very insensitive. I am surprised the military does not use it... I am actually giving away one of my patent ideas.
It has to be the safest primary...Good observation Marx. Bert has a horror story with SADS static... but it may of been exposed to light or made at
the wrong temperature.
It is the perfect primary. Really anxious to hear more electric test from you.
|
|
|
MineMan
National Hazard
   
Posts: 998
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
In fact, we should all write a patent together to replace LA in commercial and military caps...
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1335
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
If is something for commerce purpose, the price is more important, than pure living environment. Therefore is (almost) all detonators with lead. If
should price Ag and Pb should same, will in almost all detonators SADS.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
markx
National Hazard
   
Posts: 645
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Unfortunately Liptakov is right about cost being the main driving feature behind commercial applications. But that does not mean that a discovery is
unusable just because of higher cost, there are fields of application where cost is not imperitive, but rather the fact that a function is fulfilled
or a specific property of a substance is present. Like aerospace, military or some other niche applications.
Taking a patent application is easy, but before that, one has to be rather sure that there is a viable practical interest behind it all. Otherwise it
is just a rather big hole in your wallet caused by that document that nonone wants to buy in the end. And it is quite a cost to cover really just for
applying for and keeping patent protection over a particular application. I know because I have several patent references in the electochemical
energy storage field and went through the whole process.
For now I'm just happy to have a reasonably usable solution for hobbyist experimentation purposes....as much as what we engage in can be called
reasonable after all 
About the electrical sensitivity testing....well yeah, I do need to construct a device that can store a variable predetermined amount of electrical
energy and then release it through the sample under testing to get a quantitative result. It involves quite a bit of mechanical and electronical work,
but it is doable. Zapping samples with piezo discharge or the kind of zap box that I used and making statements about the sensitivity or
nonsensitivity of the sample is not going to hold water in any way. So again I must remind that my results are at the monent singular, preliminary
and by no means should
they be translated as a green light to handle DS without concern to electrostatic dangers.
Exact science is a figment of imagination.......
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
@Mineman, LL and markx,
Not only the price is important but also:
-the efficiency of reliable D2D (from shock, flame or electric spark)
and
-the initiating ability...for example how much Lead azide is required to set off TNT vs SADS?
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
markx
National Hazard
   
Posts: 645
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Quote: Originally posted by PHILOU Zrealone  | @Mineman, LL and markx,
Not only the price is important but also:
-the efficiency of reliable D2D (from shock, flame or electric spark)
and
-the initiating ability...for example how much Lead azide is required to set off TNT vs SADS? |
Of course....I was speaking on a generic level assuming comparable performance of a device/solution.
BTW Philou, how are your attempts on the sand crush test going? Any luck of seeing comparative data on the SADS mixtures? It would be really
interesting to compare the results 
My new coarser samples of limestone sand also arrived this week, but the granulometry is different at the upper end of grain size distribution. I
guess I will try a whole repetition of the series with a single batch of the new sand to limit the uncertainty factor and see what comes out of it.
Exact science is a figment of imagination.......
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1335
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
SADS
However, heads up ! For amateur use is SADS clearly best material. Ag + HNO3 is available ( against NaN3). And price pure fine powder of Ag? How
much cost 20 poison cigarets ? Same as 3 - 4 grams of non-poison Silver. And still, from 4g Ag is possible prepare 4,8g SADS. Is one of you a smoker?
Throw away your cigarettes and buy a beautiful, clean and noble metal....Dr.
https://www.youtube.com/watch?v=jUZMiLmfaws
[Edited on 4-3-2017 by Laboratory of Liptakov]
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
MineMan
National Hazard
   
Posts: 998
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
As being part of the blasting industry I disagree. Not long ago I was on a loaded blast pattern when an excavator almost (less than 1" away) ran over
a surface cap that was connected to all of the holes.
They already pay $30 a cap... I don't think .1g of silver per cap would change that much... safety is king with the mining culture... they pride
themselves in it... on mine I worked at one could not enter a pickup bed truck without fall protection.
Remember, they only use 120mg of this stuff to fire PETN... we as armatures can make that amount for nearly 10 cents.
Accidents and investigations cost a lot... If a company like orica announced a switch to a infallible primary explosive, because of the strong safety
culture in the industry their stock would go up, and all competitors would have to follow.
[Edited on 4-3-2017 by MineMan]
|
|
|
markx
National Hazard
   
Posts: 645
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Quote: Originally posted by MineMan  | As being part of the blasting industry I disagree. Not long ago I was on a loaded blast pattern when an excavator almost (less than 1" away) ran over
a surface cap that was connected to all of the holes.
They already pay $30 a cap... I don't think .1g of silver per cap would change that much... safety is king with the mining culture... they pride
themselves in it... on mine I worked at one could not enter a pickup bed truck without fall protection.
Remember, they only use 120mg of this stuff to fire PETN... we as armatures can make that amount for nearly 10 cents.
Accidents and investigations cost a lot... If a company like orica announced a switch to a infallible primary explosive, because of the strong safety
culture in the industry their stock would go up, and all competitors would have to follow.
[Edited on 4-3-2017 by MineMan] |
I don't oppose your train of thought, but first of all there is no such thing as an infallible solution, even more so for something as touchy in
nature as a primary can be regarded. And it all depends on how big a picture the people in charge are able to see. And what they regard as being part
of "the cost" for things or processes. From what I have seen so far, they tend to run blind by the numbers and abandon reason for the sake of profit.
Enforcing safety culture is on the rise lately, yet also this field is governed by cost and mostly structured in a way that serves more to protect the
skin of those in charge and not the ones in danger of getting hurt. It is all about diverting cost and responsibility.
For example.....we have to take a written permission from the head of maintenance and operations if we want to step on a chair to dust off the top
shelf in the lab because it is regarded as "working in height" by corporate procedure. And it is required to dust off the top shelf every two weeks by
5S corporate procedure. If we fail to take the permission and fall off the chair and break our neck, then it is the local manager who gets the boot
for not following procedures and not the corporate head of safety. We lock ladders now...to the wallstand in the workshop.....not the ones that lead
to the silo towers, because, oh surprise, it costs too much, but the ones you use to change a lightbulb in the hallway. Diverting responsibility under
the slogan of raising safety culture.....and standing ovations follow.
We produce an assortment of cheap products that have near zero profit margin and cost twice the value of the cargo to ship to neighbouring countries
on truck. But oh joy....we can change the cost center that handles the transportation and write that loss off of our production site budget and dilute
it into a bigger pot. Diverting of cost under the slogan of restructuring the logistics....yet again standing ovations and a prize for outstanding
production efficiency, plus the stock goes up.
A production site was sold to competing corporation for the sake of cost saving....disregarding the fact that this site was holding the largest and
most sophisticated R&D facility with research equipment and the brightest heads in the field. All of which is now lost and has to be outsourced
for high cost....and the top managment wonders how come it takes now three times longer to complete a project and no deadlines are met.
I guess what I'm trying to say with all of this is that everything is always ruled in the end by cost or more precicely "the summary cost perceivable
by the ones in charge", no matter under what kind of a slogan of promoting higher values and goals it is advertised at the front end of things.
Exact science is a figment of imagination.......
|
|
|
| Pages:
1
..
4
5
6
7
8
9 |