John Jijadi
Harmless

Posts: 4
Registered: 26-1-2011
Member Is Offline
Mood: No Mood
|
|
resonance structure of benzoate ion
Can the lone pair electrons of oxygen in benzoate ion delocalized into benzene ring?
|
|
|
peppovsky
Harmless

Posts: 23
Registered: 5-12-2009
Member Is Offline
Mood: No Mood
|
|
No, since for this to happen, you will have to get the lone pair electrons to add to the carbon-oxygen bond (thus forming a double
bond), and with this transfer of electrons you have two options (delocalized electrons can't go farther than atom-to-bond) :
A. Take electrons from the double bond of the other carbon-oxygen into the oxygen (a property of the carboxylate ion which makes it more stabilize,
but helps you not with the "electrons into ring" issue).
B. Having the electrons from the already-double-bonded oxygen creating a carbon-carbon bond with an aromatic carbon... which leads to a five-bonded
carbon, in whatever way you look at it. A big no no.

[Edited on 27-1-2011 by peppovsky]
[Edited on 27-1-2011 by peppovsky]
|
|
|
spirocycle
Hazard to Others
  
Posts: 197
Registered: 29-9-2010
Member Is Offline
Mood: No Mood
|
|
but remember that the lone pair IS delocalized among the oxygen atoms, which are conjugated with the ring system
There will likely be some partial overlap between the P orbitals of the oxygen and the pi bonds in the ring
|
|
|
peppovsky
Harmless

Posts: 23
Registered: 5-12-2009
Member Is Offline
Mood: No Mood
|
|
overlap of orbitals is not resonance, nor delocalizing.
[Edited on 27-1-2011 by peppovsky]
|
|
|
madscientist
|
Thread Moved
27-1-2011 at 20:02 |
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
To quote from p 447 of Anslyn and Dougherty's Modern Physical Organic Chemistry:
| Quote: |
The negative charge of benzoate anion is not in conjugation with the aromatic ring, and so it cannot be stabilized by resonance.
|
btw, peppovsky, you're using the wrong type of arrows for resonance 
[Edited on 1-28-11 by DDTea]
"In the end the proud scientist or philosopher who cannot be bothered to make his thought accessible has no choice but to retire to the heights in
which dwell the Great Misunderstood and the Great Ignored, there to rail in Olympic superiority at the folly of mankind." - Reginald Kapp.
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DDTea  | To quote from p 447 of Anslyn and Dougherty's Modern Physical Organic Chemistry:
| Quote: |
The negative charge of benzoate anion is not in conjugation with the aromatic ring, and so it cannot be stabilized by resonance.
|
btw, peppovsky, you're using the wrong type of arrows for resonance 
|
Your "resonance" does not exist, it is just fiction (or trick if you like).
In chemistry fiction cannot stabilize anything. It is possible only on paper, in more or less shitty books, equally modern and old.
Do not mess physical delocalization of electrons with (not existing in reality) "resonance".
|
|
|
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
Of course "resonance" is a fiction; it's a model (well, the result of a model really). No model is perfectly accurate, but they're accurate enough to
give good results. If they aren't accurate enough to give meaningful/usable results, then you switch to a different, often more complicated model. A
hybrid valence bond/MO approach is sufficient to answer this question (as is used in a lot of A & D's book). There's no need to be pedantic with
a simple, likely sophomore O. Chem question, though--that distracts from the answer.
Of course, if you really want to, you could go and calculate the molecular Hamiltonian or go Computational on this, but that approach isn't very
useful when it comes to solving O. Chem problems 
By the way, I'd hardly call Anslyn & Dougherty's book "shitty," but if you feel you can write a better one, go ahead.
[Edited on 1-29-11 by DDTea]
"In the end the proud scientist or philosopher who cannot be bothered to make his thought accessible has no choice but to retire to the heights in
which dwell the Great Misunderstood and the Great Ignored, there to rail in Olympic superiority at the folly of mankind." - Reginald Kapp.
|
|
|
thedy
Harmless

Posts: 5
Registered: 6-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by peppovsky  | No, since for this to happen, you will have to get the lone pair electrons to add to the carbon-oxygen bond (thus forming a double
bond), and with this transfer of electrons you have two options (delocalized electrons can't go farther than atom-to-bond) :
A. Take electrons from the double bond of the other carbon-oxygen into the oxygen (a property of the carboxylate ion which makes it more stabilize,
but helps you not with the "electrons into ring" issue).
B. Having the electrons from the already-double-bonded oxygen creating a carbon-carbon bond with an aromatic carbon... which leads to a five-bonded
carbon, in whatever way you look at it. A big no no.

[Edited on 27-1-2011 by peppovsky]
[Edited on 27-1-2011 by peppovsky] |
I have two questions.
First:How is it possible,that electrons can't go farther than atom-to-bond?After all,this is the princip of the resonance effect,isn it?In phenol,for
example electrons move through the whole benzene ring...
And second:
You wrote:,, Having the electrons from the already-double-bonded oxygen creating a carbon-carbon bond with an aromatic carbon... which leads to a
five-bonded carbon, in whatever way you look at it. A big no no."
But carbon will be not five-bonded,because electrons from carbon ring will move,like in phenol or other examples.Or am I wrong?
Thanks
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Have a look at page 9, all you want to know 
http://www.auburn.edu/~deruija/pda1_acids1.pdf
|
|
|
thedy
Harmless

Posts: 5
Registered: 6-2-2012
Member Is Offline
Mood: No Mood
|
|
Here,carboxylic group conjugate to ring: http://www.google.sk/imgres?q=benzoic+acid%2Bresonance+struc...
And carbon isn t 5-bonded,because electrons move to adjacent carbon and so on.....Am I right?
[Edited on 6-2-2012 by thedy]
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
No, see my post above.
[Edited on 6-2-2012 by ScienceSquirrel]
|
|
|
thedy
Harmless

Posts: 5
Registered: 6-2-2012
Member Is Offline
Mood: No Mood
|
|
I have read this article.But I still understand.Here is link,why: https://docs.google.com/drawings/d/1B7CAXUEJ8Grs3Ci8W_GfvLPB...
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Moving the electrons from the double bond would give you a positively charged oxygen and a negative charge on a ring carbon.
This would be a highly unstable structure.
Have a look at the following tables. 3 and 4- Nitrobenzoic acids are weaker than 2- Nitrobenzoic which is due to the neighbouring group effect, like
chloroacetic acid compared with acetic acid.
2 and 4-Nitrophenols are stronger than 3-Nitrophenol, resonance effect.
Formula Name Temp pKa
C7H5NO4 2-Nitrobenzoic acid 18 2.16
C7H5NO4 3-Nitrobenzoic acid 25 3.47
C7H5NO4 4-Nitrobenzoic acid 25 3.41
C6H5NO3 2-Nitrophenol 25 7.17
C6H5NO3 3-Nitrophenol 25 8.28
C6H5NO3 4-Nitrophenol 25 7.15
[Edited on 7-2-2012 by ScienceSquirrel]
|
|
|
arsphenamine
Hazard to Others
  
Posts: 236
Registered: 12-8-2010
Location: I smell horses, Maryland, USA
Member Is Offline
Mood: No Mood
|
|
According to a single point energy computation using GAMESS-US, the partial charges on atoms don't change hugely between neutral acid and anion.
The charge plot estimates that only the 1-C ring atom experiences significant electron density decrease by simple proximity to the carboxylic moiety,
as do the ortho ring carbons to a lesser extent.
If you reproduce this calculation, be warned that each one took about an hour on a 6-core SMP machine.
|
|
|
thedy
Harmless

Posts: 5
Registered: 6-2-2012
Member Is Offline
Mood: No Mood
|
|
Thanks for answers 
|
|
|
arsphenamine
Hazard to Others
  
Posts: 236
Registered: 12-8-2010
Location: I smell horses, Maryland, USA
Member Is Offline
Mood: No Mood
|
|
Beating a dead horse:
The acidic carboxy function has its own PI orbital system.
The anion's PI system delocalizes over the whole ring.
Benzoic Acid
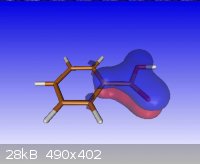
Benzoate anion
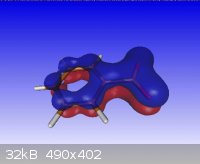
Note: the single point energy eigenvalues were produced by MOPAC2009, from OpenMopac
[Edited on 10-2-2012 by arsphenamine]
|
|
|
thedy
Harmless

Posts: 5
Registered: 6-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by arsphenamine  | Beating a dead horse:
The acidic carboxy function has its own PI orbital system.
The anion's PI system delocalizes over the whole ring.
Benzoic Acid
Benzoate anion
Note: the single point energy eigenvalues were produced by MOPAC2009, from OpenMopac
[Edited on 10-2-2012 by arsphenamine] |
So that means,carboxyl group is in conjugation with ring?Benzene can take pi electrons from carboxyl group?If yes,that means,that benzoic acid is
stronger acid like for example formic acid,because negative charge is stabilized over whole ring----more stable,Is it true?
[Edited on 15-2-2012 by thedy]
[Edited on 15-2-2012 by thedy]
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Quote: Originally posted by thedy  | Quote: Originally posted by arsphenamine  | Beating a dead horse:
The acidic carboxy function has its own PI orbital system.
The anion's PI system delocalizes over the whole ring.
Benzoic Acid
Benzoate anion
Note: the single point energy eigenvalues were produced by MOPAC2009, from OpenMopac
[Edited on 10-2-2012 by arsphenamine] |
So that means,carboxyl group is in conjugation with ring?Benzene can take pi electrons from carboxyl group?If yes,that means,that benzoic acid is
stronger acid like for example formic acid,because negative charge is stabilized over whole ring----more stable,Is it true?
[Edited on 15-2-2012 by thedy]
[Edited on 15-2-2012 by thedy] |
pKa Formic acid 3.77
pKa Benzoic acid 4.21
[Edited on 15-2-2012 by ScienceSquirrel]
|
|
|
arsphenamine
Hazard to Others
  
Posts: 236
Registered: 12-8-2010
Location: I smell horses, Maryland, USA
Member Is Offline
Mood: No Mood
|
|
| Quote: | | So that means,carboxyl group is in conjugation with ring?Benzene can take pi electrons from carboxyl group?If yes,that means,that benzoic acid is
stronger acid like for example formic acid,because negative charge is stabilized over whole ring----more stable,Is it true? |
More stable electronically doesn't mean a stronger acid.
A 4.19 pKa means the benzoate Gibbs free energy (= -RT ln(Ka)) is 23.9 kJ/mol or ~5.7 kCal/mol, about 4x the energy in a hydrogen bond.
The enthalpy of formation is a bit higher which means there is a significant entropy change when the anion forms.
|
|
|