DrDevice
Hazard to Self
 
Posts: 74
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
Hofmann rearrangement via chloroamide not working?
Hi all,
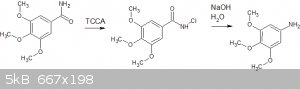
some time ago I outlined a procedure to use the Hofmann rearrangement to convert from 3,4,5-trimethoxybenzamide to 3,4,5-trimethoxyaniline, using
household bleach as the halogen source:
https://www.sciencemadness.org/whisper/viewthread.php?tid=64...
Recently I have tried a Hofmann rearrangement on the same compound, but with isolation of an intermediary chloroamide prior to the actual
"rearrangement" step. I believe the chloroamide step worked OK, but I've had an unexpected result with the final step. I've provided my procedure
below, comments and suggestions welcome.
The chloroamide synthesis is based on the procedure in paper "Preparation of N-chloroamides using trichloroisocyanuric acid", Hiegel, Hogenauer,
Lewis, 2005. See attached.
10g (47mmol) 3,4,5-trimethoxybenzamide was added to a solution of 4.5g (19.2mmol) of TCCA in 100ml of methanol with magnetic stirring. The molar
ration of TCCA was based on the 1.1 equivalents of chlorine suggested by the paper, adjusted for the "90%" purity of the pool grade TCCA I was using.
A brief mildly exothermic reaction occurred, and after a few minutes a precipitate of cyanuric acid formed.
Stirring continued for an hour, after which the solution was filtered. The MeOH was evaporated, leaving a pale creamy solid.
This solid was recrystallised from boiling toluene, with hot filtering to remove some insoluble material. Pre-heating of the funnel etc is necessary
as the product crystallises out readily as temperature drops.
After refrigeration for a few hours, the product is filtered with suction and dried at low temperature, leaving a white solid.
MP was measured at 142C - 143C, quite sharp. I unfortunately don't have a literature value to compare it with. TLC with 3:1 EtOAc:Hexane gave an Rf of
0.67, compared with a streak from 0.09 to 0.32 for the benzamide.
I am assuming this is the n-chloroamide (but I don't know for sure).
The paper suggests (without a lot of useful experimental references) that this can then be converted to the aniline with aqueous NaOH.
I attempted this step with 2.46g of the (assumed) n-chloroamide = 10mmol. I added this to a solution of 2.4g NaOH in 100ml of water. I refluxed this
for 22 hrs (I checked with TLC a couple of times during the reaction but I'm not confident with the results I obtained ). The solution briefly had a
pink colour before going colourless. I also noted an ammonia smell during reflux.
On cooling, fine needle crystals were precipitated. These however had a melting point of 170 - 171C, compared to the literature value for
3,4,5-trimethoxyaniline of 110C - 113C. So not even close, but sufficiently different from the source material to believe something had happened. BTW,
the source benzamide MP is 176C - 177C.
TLC with 1:1 EtOAc:Hexane gave Rf of 0.7 vs 0.21 for the chloroamide.
Something happened - but what??
Attachment: hiegel2005.pdf (135kB)
This file has been downloaded 317 times
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Do you still have some "chloroamide" to test?
When chloroamides are cautiously heated they melt, then quickly start bubbling and turn black. At least in my hands and melting point capillaries.

Another very characteristic property of these compounds is that in acidic (acetic acid) solution they set free I2 from KI. The reaction is
instantaneous and quantitative, it can be used for the determination of purity of these compounds!
Edit:
Trimethoxybenzoic-acid melts around 171-172 C. It seems close. 
You used 6x molar ammount of NaOH for the rearrangement. It is unnecessary, but can contribute to hydrolysis. For a Hofmann rearrangement you
theoretically only need 2x molar ammount of NaOH compared to the chloroamide, if my memory is correct. So in theory 2.2-2.5x molar ammount of NaOH
should be enough.
When I used this type of rearrangement on a similar scale as you did, I dissolved the chloroamide in a VERY COLD NaOH solution, around -5 C, then let
it slowly heat up to room temp, then started further heating up to 80 C. In my case the reaction "kicked-in" around 12 C : the colour of the
originally water clear solution started to turn more and more yellowish-orangish. The solution was held at 80C for a few minutes only, then heating
was stopped. The whole process took me less than three hours, but I was obviously over-cautious at the beginning. Ooops, there was continuous stirring
too!
Please keep us informed about this experiment! I'm very interested in your results because you do something very similar (on a different substrate) to
my experiments from a few weeks ago!
[Edited on 25-9-2019 by Pumukli]
[Edited on 25-9-2019 by Pumukli]
|
|
|
DrDevice
Hazard to Self
 
Posts: 74
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
Well, I've had zero success with getting the aniline. Each time I am ending up with an amorphous white solid with a MP of 134 - 135C, no matter if I
vary length of time, reflux vs heating, NaOH concentration etc.
So I've given up for now. I am having reasonable success getting the aniline directly from the benzamide using TCCA; see:
http://www.sciencemadness.org/talk/viewthread.php?tid=154422
Thanks for the suggestions anyway.
|
|
|
|