hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
Potassium Ferricyanide + Ammonia - Fire/Explosion Hazard?
http://www.ihcworld.com/royellis/ABCSafe/chemicals/potassium...
Potassium ferricyanide is incompatible with:
Acids forms hydrocyanic acid.
Ammonia fire and explosion hazard.
Heating produces toxic fumes of hydrocyanic acid.
Okay, I understand the poison hazards given the presence of CN in the formula. But I'm having a difficult time imagining how this substance could
form something that is a "fire and explosion hazard" when mixed with ammonia.
I remember making some tetraammine copper salts that had explosive (or at least deflagration) properties years ago. Maybe something similar here? As
I recall though, all the energetic tetraaimmine salts had oxygen to combine with the the hydrogen in ammonia (i.e. they were nitrates or at least
sulfates).
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I assume that information is from an MSDS. Usually that kind of sheets errs on the safe side in the extreme.
From personal experience I can say that ferricyanides are quite stable. The ferricyanide is mildly oxidizing (a property, which is used in photography
in some processes). I have had it mixed with all kinds of chemicals, including ammonia. No dangerous reaction occurred, actually no reaction occurred
at all, not even when heated.
With acids, there might be release of HCN, but you need rather harsh conditions for that. Mixing it with e.g. 10% HCl does not produce copious amounts
of HCN. You need to add the compound to concentrated acids and need to heat the solution in order to get a decent amount of HCN.
Ferrocyanide is more stable than ferricyanide, but both chemicals can be safely used in a home lab and their toxicity is quite low.
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
When I was a teen, I had a chemistry set that had potassium ferrOcyanide (not ferrIcyanide). I remember the caution in the set for that chemical said
"Do not mix with an acid or expose solution to sunlight". I was curious about the sunlight thing, so I took a test tube full of solution outside and
left it in the sun for a couple hours. As I recall, the solution darkened (perhaps to orange?). I was so scared that I had made cyanide that I was
afraid to touch the test tube. I hosed it down with a garden hose.
FerrIcyanide is obviously exposed to light (though in the presence of an organic iron salt, not in a solution) in the cyanotype process, which I have
done and posted pictures from here before.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Ferrocyanide is even more stable than ferricyanide. In the EU (and I think in many other places as well) it is used as an additive in some food stuff
to avoid clumping together, due to absorption of moisture. Even in your stomach, which is quite acidic, it does not decompose noticeably to HCN and
ferrous salts.
The ferrocyanide and ferricyanide indeed are light-sensitive (the ferricyanide much more than the ferrocyanide). There indeed is application in
photographic processes. I myself have played around with cyanotype photography as well. With this process, I never had any issues with production of
HCN.
Just use common sense with these chemicals. Do not put them in warm concentrated acids and even if you do and some HCN is formed, if you do this
outside and you use small quantities (test tube scale), then there is no real risk as long as you don't stick your nose in the tube.
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Well, I also used ferricyanide in photo processing. I was inventing some chemical process for black and white paper when I was a child and used some
acidic bath to stop an action of developer and then potassium ferricyanide bath as a first step to invert the image (my goal was to get a positive
image by direct exposition on a photographic paper).
I kept all the solutions and papers on a table and was trying to improve my process for the whole day.
At the evening the smell of HCN was quite strong. I was afraid a bit, but the point is that HCN has a very good detection level. If you mix
ferri/ferrocyanide with an acid you always notice that smell. Even if they are mixed in a drain. If you not prepared you can feel uncomfortably, even
can have some type of panic attack (all these cases can train you to distinguish the panic attack from a real case of poisoning which mostly occurs
when you are totally calm).
I am thankful to woelen who explained that the amount of the poison from such reaction is negligible.
[Edited on 7-10-2019 by teodor]
[Edited on 7-10-2019 by teodor]
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Some people are very sensitive to HCN and can smell it very well, but it is known that appr. one third of people cannot smell HCN (this is genetically
determined). I am one of them. I once made some HCN on purpose (from a small spatula of KCN and some luke warm acid) and wafted it towards my nose. No
smell at all. As a test to be sure that my method of wafting works, I made some Cl2 in similar amounts and wafted that towards my nose. I was greeted
with a strong smell of chlorine. So, my conclusion is that I cannot smell HCN.
Then I destroyed the HCN by lighting it. It burns quite well, just keep a flame near the open end of the test tube.
|
|
|
wg48temp9
National Hazard
   
Posts: 761
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by woelen  | Some people are very sensitive to HCN and can smell it very well, but it is known that appr. one third of people cannot smell HCN (this is genetically
determined). I am one of them. I once made some HCN on purpose (from a small spatula of KCN and some luke warm acid) and wafted it towards my nose. No
smell at all. As a test to be sure that my method of wafting works, I made some Cl2 in similar amounts and wafted that towards my nose. I was greeted
with a strong smell of chlorine. So, my conclusion is that I cannot smell HCN.
Then I destroyed the HCN by lighting it. It burns quite well, just keep a flame near the open end of the test tube. |
In the past I had a bottle of KCN. If I uncapped the bottle I could smell it within seconds in the small garden shed I did my experiments then.
I used to eat the core of apples. I figured the extra fiber was good. If I chewed the seeds they would remind me of the smell of KCN and they where
very bitter. I now know that apple seeds contain amygdalin, which is converted into cyanide when the seeds are chewed or crushed.
I guess I am one of those that can smell HCN.
Amygdalin
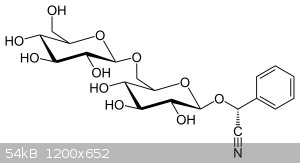
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Sometimes smells of some chlorinated compounds is very close to that of HCN for me, also it is not possible for me to distinguish smell of
concentrated H2SO4 and HCl (when I put some carbonate inside and there is a spray in the air), also HCl can smell differently in different
concentrations and till now I unable to get any agreement from myself how H2S actually smells - most of the time it smells for me as a burning rubber,
not even close to eggs. And possible there are compounds I couldn't smell at all.
Hot H2SO4 smells for me exactly as sugar. I don't know whether is it because sugar is processed with sulfur oxides or by some other reason.
[Edited on 8-10-2019 by teodor]
[Edited on 8-10-2019 by teodor]
|
|
|