finetune
Harmless

Posts: 14
Registered: 8-10-2019
Member Is Offline
|
|
Konrblum oxidation of 2-(chloromethyl)-2-methyl-1,3-dioxolane
I want to try the Kornblum oxidation of 2-(chloromethyl)-2-methyl-1,3-dioxolane (CMD), CAS 4469-49-2, to 2-methyl-1,3-dioxane-2-carbaldehyde (MDC),
CAS 39050-39-0.
Sadly, I did not find any data (mp, bp, density, etc) for MDC, can anybody please help me?
Any other procedure from the halogen to the aldehyde is highly appreciated.
CMD is prepared by the chlorination of acetone in ethylene glycol
J. org. chem. Vol. 46, 1981, 2532-2538 pdf
For the Kornblum I plan to dissolve CMD in excess DMSO, add equimolar triethylamine and reflux. Maybe NaHCO3 works aswell, I will have to try.
Thank you very much,
Finetune
CMD structure:
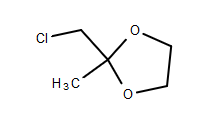
MDC structure:
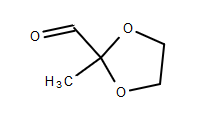
[Edited on 9-10-2019 by finetune]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
https://en.wikipedia.org/wiki/Sommelet_reaction
|
|
|
finetune
Harmless

Posts: 14
Registered: 8-10-2019
Member Is Offline
|
|
Thanks, but the acidic hydrolysis in the Sommelet may interferes with the ketal in CMD
|
|
|
Cactuar
Harmless

Posts: 32
Registered: 25-7-2014
Location: Denmark
Member Is Offline
Mood: No Mood
|
|
Ketals are actually quite resilient being bidentate. The sommelet reaction will give you an iminium cation which I would guess will react much more
rapidly using a weak acid.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Quote: Originally posted by Cactuar  | | Ketals are actually quite resilient being bidentate. The sommelet reaction will give you an iminium cation which I would guess will react much more
rapidly using a weak acid. |
I agree, the sommelet reaction is just so easy.
Tried forming the adduct in ethanol from benzyl chloride and hexamine, but the intended delepine I had in mind hasn't happened afterwards when I used
the adduct, I ended up with a very high yield of benzaldehyde instead of benzylamine.
|
|
|
finetune
Harmless

Posts: 14
Registered: 8-10-2019
Member Is Offline
|
|
Thanks!
After reading more about the ketals of (chloro-)acetone, it seems they are quite stable in dilute acid at RT. [J. Prakt. Chem. 156, 103-148 (1940),
doi: 10.1002/prac.19401560402]
As I have hexamine on hand, I will try the Sommelet at first but it really depends on the yields.
The DMSO for the Kornblum is good 14 mol/L really cheap and the triethylamine is regeneratable (if one really needs TEA).
The hexamine is about 7.1mo/kg and more expensive.
I will post a synthesis of the chloroketal and the aldehyde anyway. Currently I am optimizing the conditions for the chloroketal synthesis from
acetone and TCCA in ethylene glycol solvent.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
But keep in mind the kornblum oxidation stinks horribly.
|
|
|
finetune
Harmless

Posts: 14
Registered: 8-10-2019
Member Is Offline
|
|
Yes, I thought about leading gaseous dimethylsulfate into a flame. H2O2 may work aswell, I will have to try.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
I used simply NaOCl bleach, the stuff from the supermarket with I think 3,8%.
|
|
|
finetune
Harmless

Posts: 14
Registered: 8-10-2019
Member Is Offline
|
|
Thanks! Totally forgot about NaOCl!
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Some data on the aliphatic Sommelet:
https://pubs.rsc.org/-/content/articlelanding/1953/jr/jr9530...
It appears:
strong acid + low heat -> Delepine
weak acid + high heat -> Sommelet
but the latter is incompatible with enolizable aldehydes. Your aldehyde is not enolizeable -- I am not sure if the usual workup conditions (50%
H2O/GAA, reflux) would destroy the ketal.
I should also mention, if triethylamine is available, triethylamine N-oxide can probably be used to prevent release of any DMS. The amine oxide acts
as both base and oxidant and is more reactive than DMSO itself. See the attached paper (which uses trimethylamine N-oxide).
Attachment: godfrey1990.pdf (103kB)
This file has been downloaded 334 times
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
finetune
Harmless

Posts: 14
Registered: 8-10-2019
Member Is Offline
|
|
Thank you for the information, I will have a look into it.
|
|
|