Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
How do you do research for organic procedures?
I understand the reaction mechanisms and reagants and all, but in practice doing reactions takes experience. Workup is the part i dont understand.
I'd also like to find more procedures for getting organic substances from OTC sources (i once found a procedure for making butyric acid from butter,
for example)
One thing i'm interested in is making esters. I know how fisher esterification works, but workup is the tricky part. So you mix together a pure
alcohol and a pure carboxylic acid, you end up with a mixture of product, alcohol, acid, and water, how do you workup to isolate the ester. Is it just
a solubility extraction because the ester is the only component of mixture soluble in ether? Where would you find solubility data to decide the best
solvent to separate the ester?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
If you extract the mixture with dilute carbonate solution, that neutralizes the acid. The alcohol is usually much more soluble in water than the
ester, so you have mostly ester left in the organic layer.
I've had a great deal of difficulty with a lot of esters, though, so i may not be the one to ask (you know how many times I've tried making butyl
salicylate? And it's NEVER worked for me!).
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Quote: Originally posted by DraconicAcid  | If you extract the mixture with dilute carbonate solution, that neutralizes the acid. The alcohol is usually much more soluble in water than the
ester, so you have mostly ester left in the organic layer.
I've had a great deal of difficulty with a lot of esters, though, so i may not be the one to ask (you know how many times I've tried making butyl
salicylate? And it's NEVER worked for me!). |
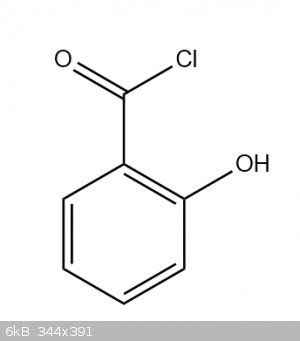
Could you use thionyl chloride to make this, and use it for esterification?
https://link.springer.com/article/10.1134%2FS107036321509026...
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I don't have thionyl chloride, and despite my fully equipped college lab, current legislation makes it a real pain in the neck to get. I do have a
prep for the chloride from the aldehyde (using chlorine that I can make), but that will wait until I have a bigger stretch of time in which to play
around.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Finding the correct workup procedure is not easy, and one way to learn is by reading experimental sections of various papers. After a while you start
to see what is the usual practice for a certain type of reaction.
For esters, usually you have to add an acid catalyst, such as H2SO4, to accelerate the esterification. Remember that a catalyst accelerates both the
forward and reverse reaction. So the first step in the workup is to neutralize the acid to prevent the reverse reaction (hydrolysis) which destroys
your ester product. You don't want to neutralize too much, because basic conditions also hydrolyze esters. Then you can extract the ester with a
solvent, or distill the ester from the reaction mixture, ...
|
|
|