numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
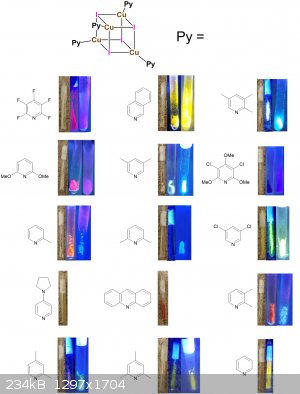
An assortment of fluorescent pyridine copper iodides
Based on the well known paper in chemical education (J. Chem. Educ. 2012, 89, 7, 946-949), I reckon that the fluorescence thermochormism of pyridine
copper iodide is more or less a known phenomenon among the home chemist community. Especially after it was popularized by nurdrage, and I believe
Woelen has a page discussing the complex on his website as well.
With a little spare time, my girlfriend and I have compiled a nice assortment of copper iodide complexes using random pyridines I had laying around.
The synthesis is given in the SI of the aforementioned paper, and involves adding a MeCN solution of CuI, KI, and acid, to a solution of pyridine.
Some of the proposed complexes below are unpublished, but since this was just for fun I didn't characterize them. I'm pretty sure the proposed
complexes are accurate for most of them, but not all of them. For example in the 4-aminopyridine case, there's a reasonable chance that it's
coordinating with the exocyclic nitrogen (maybe that's why non-fluorescent?)
Every sample was ampuled, and displayed under ambient light, 365 nm, and 365 nm at -196 °C respectively. The acridine and aminopyridine samples were
non-fluorescent even at low-t's so pics were not taken.
Maybe someone can find a discernible pattern. Seems like there is no clear trend sterically or electronically - maybe something to do with the crystal
packing since they are not fluorescent in solution. I've also included side by side pictures so the relative brightness is more apparent. Either way,
enjoy the pretty colors!
 
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I have done this experiment with pyridine and with morpholine. I could obtain yellow, green and orange fluorescence with CuI. Quite interesting stuff.
I unfortunately don't have access to all those ligands you show in your pictures, but it is very interesting to see that there are many closely
related ligands, which have different colors of fluorescence.
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Wow really nice work! Some look really gorgeous like the isoquinoline and 3,5-dichloropyridine ones. How did you manage to get the ligands, some look
really expensive and difficult to make?
|
|
|
numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Heptylene  | | Wow really nice work! Some look really gorgeous like the isoquinoline and 3,5-dichloropyridine ones. How did you manage to get the ligands, some look
really expensive and difficult to make? |
Yea, those were unusually bright, interestingly the pentachloropyridine formed no complex at all, was expecting that one to be nice too...
Ligands are from all over. Some were made, like the trimethoxy-dichloro one I made using SNAr with 2,4,6-trifluoro-3,5-dichloropyridine. But the vast
majority are from a defunct lab that was going to scrap them.
|
|
|
|