B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Accidental synthesis of fluorescence polymer
Hey there, just wanted to say that English isn't my native language. I apologize if my styling is a bit odd or if my grammar is a bit off.
Abstract:
During the distillation of benzyl chloride, polymerization had occurred. The polymer fluorescence green under a UV light as seen below:
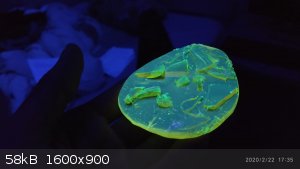 
The polymer is suspected to be "polybenzyl"[1].
What happened:
Preparation of Benzyl chloride:
-To a 1L erlenmeyer flask, I first added 600gr of HCl(aq)(wt=32.4%), followed by 200gr of BnOH(l). The mixture was homogeneous.
-I stirred and heated the mixture untill it started refluxing. I kept it refluxing for about 2-3 minutes.
-After the stirring ceased two layers had formed. I kept the top layer.
-I washed the top layer twice with NaHCO3(aq) and once with NaCl(aq). I kept the bottom layer(in all three washes).
-I dried the solution with CaCl2(s). When the solution became lucid I decanted the liquid into a fresh erlenmeyer flask.
Distillation and the polymerization of benzyl chloride
-I prepared the following setup:
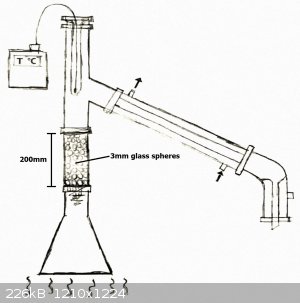
-I've heated the solution to a boil. At first everything went fine, but not much after, the condensed liquid created a blockage inside of the column
and pressure started building up. The condensed liquid started boiling inside of the column and the glass spheres started to "pop" intermittently.
-I've noticed that a trace amount of copper oxide from the column have reacted with the condensed liquid. The amount of copper oxide seemed quite
negligible(it was a narrow blcakish smudge, about 0.5cm long).
-Gaseous HCl started to form. At first it was slow, but the formation soon became very rapid. I've directed the gas into a dish full of water, and
quickly turned off the hotplate.
-The mixture kept boiling for a while. Also, it slowly became more and more yellow in color.
-After a while the mixture finally stopped boiling. I knew that I've fucked up, but I was curious to see if I can save some of my BnCl. So I removed
the column and started distilling again("non-fractional distillation").
-Most of the distillate came off between 165-170C, lots and lots of HCl gas was generated and the mixture became tick and yellow(as shown below):

Attachment: 2.mp4 (1.1MB)
This file has been downloaded 271 times
-The distillate was strongly acidic and volatile. pH paper had turned completely pinkish-red(indicating pH of 1) before I got to dip it in the liquid.
-While cooling, the yellow polymer became ticker and ticker. I played with it for a while(moved it around the erlenmeyer, poured some onto a metal
dish, remelting some of the pieces and so on and so on).
My guess(after reading the research below[1]) is that the trace amount of CuCl2(which formed by the reaction between HCl and
CuO2) catalyzed a friedel-craft reaction between the armoatic rings and the benzylic carbons of the BnCl molecules. The high pressure and
temperatures probably helped too.
Here are some pictures of the polymer:
 
 
 
 
 
[1] - THE FLUORESCENCE OF POLYBENZYL - https://sci-hub.tw/https://www.sciencedirect.com/science/art...
[Edited on 22-2-2020 by B.D.E]
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Wow! What an interesting result.
I have no idea what happened but commend you on your lab skills. Careful observations during mistake moments like this that have often resulted in
scientific breakthroughs and discoveries.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I think you are right.
Polybenzyl is the most likely product.
It's important to recognise that compounds like benzyl chloride are unstable and, because the polymerisation produces HCl gas, potentially dangerous.
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
A very simply polymer synthesis indeed. I'm curious whether adding an initiator such as benzoyl peroxide, or possibly just simple UV light, would
cause the polymerization to happen as well.
The philosophy of one century is the common sense of the next.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ninhydric1  | | A very simply polymer synthesis indeed. I'm curious whether adding an initiator such as benzoyl peroxide, or possibly just simple UV light, would
cause the polymerization to happen as well. |
Probably not.
This isn't a radical reaction, it's a Friedel Crafts reaction.
Just a thought: does anyone know if you can dissolve polystyrene in benzyl chloride?
If so, can you then add a lewis acid and benzylate the styrene?
[Edited on 24-2-20 by unionised]
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Quote: Originally posted by j_sum1  | Wow! What an interesting result.
I have no idea what happened but commend you on your lab skills. Careful observations during mistake moments like this that have often resulted in
scientific breakthroughs and discoveries. |
Thanks for the kinds words man. Unfortunately it doesn't seems
that I've stumbled on anything new(although it was quite a pleasant discovery for me personally).
Quote: Originally posted by unionised  | I think you are right.
Polybenzyl is the most likely product.
It's important to recognise that compounds like benzyl chloride are unstable and, because the polymerisation produces HCl gas, potentially dangerous.
|
I agree. The gaseous HCl definitively caught me a bit off guard. Luckily it was inside of a fume hood.
Quote: Originally posted by ninhydric1  | | A very simply polymer synthesis indeed. I'm curious whether adding an initiator such as benzoyl peroxide, or possibly just simple UV light, would
cause the polymerization to happen as well. |
Although the synthesis is indeed very simple and the product is most likely to be "polybenzyl", there's a little bit more to it. There's a lot of
different MCln catalysts that prompts the polymerization of BnCl, and not all of them seems to give the exact same structure[2].
Also, the fluorescence polymers are obtainable only when the reaction is carried in the presence of oxygen[1], implying on oxidation of
some sort(study [1] suggested a possible mechanism).
In regard to the radical catalysts, I don't think it should affect the reaction too much. As the reaction most likely follows a friedel-craft
mechanism which is completely different in nature. It may however prompt the oxidation(?).
---
I've done some solubility tests in different solvent. Nothing too exciting, but if anyone is interested:
DCM - very soluble
1,4-Dioxane - very soluble
Toluene - slightly soluble
water - insoluble
Also, the polymer can fairly easily be grinned into a powder. It can also be easily remelted in the oven. At higher temperatures it decompose to a
orange "none-fluorescence"(in UV) polymer.
Solution of the polymer in dioxane:

The decomposition product alongside the original polymer.

[2] - Catalysts for the Polymerization of Benzyl Chloride - https://sci-hub.tw/https://pubs.acs.org/doi/abs/10.1021/ja01...

[Edited on 24-2-2020 by B.D.E]
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Imagine the effect of the HCl produced if it was in a sealed container.
That has been the cause of accidents.
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Oh yeah, I'd imagine it would be a disaster..
|
|
|