Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
d-limonene -> d-limonene nitrosochloride -> l-carvoxime -> l-carvone
d-limonene -> d-limonene nitrosochloride
36,5 ml of d-limonene + 30 ml of IPA (isopropanol) and magnetic stirbar were put into 500 ml Erlenmeyer flask. The flask was equipped with a
thermometer and 2 dropping funnels and was placed in ice-water bath so its content was cooled below 10 C. A mixture of 90 ml 35% HCl + 60 ml of IPA
was charged into 1 dropping funnel and solution of 15,6 g NaNO2 in 80 ml of H2O into the second one and were dropped simultaneously into the flask
(drops rate 3:1 HCl:NaNO2, water drops are slightly bigger than drops of HCl+IPA). They usually do not finish dropping simultaneously but that does
not matter. Dropping must be at such rate that the temperature is kept below 10 C (the dropping lasted 1 hour). After the addition completed the
mixture was stirred for extra half an hour with cooling in ice bath and then put into a freezer (-18 C) for extra 1 hour. At the bottom there were
crystals of NaCl and in the middle there was a semisolid green mass. Decanted NaCl and water-IPA layer quickly while the green mass was still
semisolid (on warming it solidified) and washed with cold water to dissolve remaining NaCl and then decanted again. Added 50 ml of cold 95% etanol to
the green mass, mixed it thoroughly with a glass stick and put it into a freezer (-18 C) for 2 hours with a bottle of 95% ethanol and a sinter or
Buchner funnel. Filtered quickly using vacuum and washed with freezing cold (-18 C) ethanol on the sinter until the crystals were white (50 ml of
ethanol required for washing). Air dried in a dish overnight.
Yield 18,3 g
      
[Edited on 16-3-2020 by Fery]
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
d-limonene nitrosochloride -> l-carvoxime
18,3 g limonene nitrosochloride
7,5 g urea
90 ml IPA
rfx 1 h
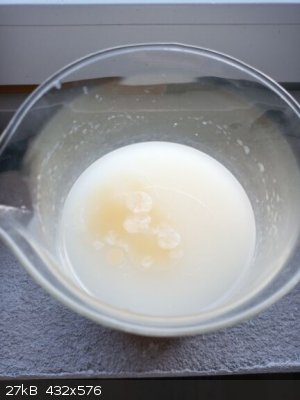
The mixture was poured into 700 ml of water where oil separated as the upper layer which later slowly solidified (few crystallization centers appeared
in few minutes which grew into a kind of flowers which merged during half an hour) and sunk to the bottom. Filtered, washed with H2O on the filter
paper and air dried overnight.
Recrystallized from 50 ml 95% ethanol, cooled down to -18 C in a freezer - it crystallizes unwillingly, after 1 day still no crystals, but after 2
days thick honey-like slurry (consistency as well color), quickly vacuum filtered on prefreezed sinter (-18 C) and washed twice with 10 ml of freezing
cold (-18 C) 95% ethanol on the sinter. Air dried in a dish overnight.
Yield 8,9 g
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Nice write-up!
|
|
|
wg48temp9
National Hazard
   
Posts: 761
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Great write up Fery. It will be very useful to me thanks.
PS: I guess the garden, forest and house work went quickly.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Nice work, ya beat me to it!
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi wg48temp9, you are right. Thanks to the quarantine - enough time to complete works outside and to perform experiments too.
|
|
|
Ripa
Harmless

Posts: 1
Registered: 10-3-2020
Member Is Offline
|
|
now that's some spicy chemistry 
|
|
|
wg48temp9
National Hazard
   
Posts: 761
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by Fery  | | Hi wg48temp9, you are right. Thanks to the quarantine - enough time to complete works outside and to perform experiments too. |
I am trapped at home under quarantine now too. So I will have time to get my garden shed lab finished but no money to buy anything.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
l-carvoxime -> l-carvone
pls correct me if you find an error here, I did these calculations decades ago
the reaction (hydrolysis) requires pH = 0,7 (pH below 0,5 and above 0,9 are both bad) - oxalic acid is used
pH 0,7 means c[H3O+] = 10^(-0,7) = 0,200 mol/l
H2OA + H2O = H3O+ + HOA-
Ka1 = 5,6 * 10^-2
Ka2 = 5,1 * 10^-5 could be neglected as it is 1000 fold lower than Ka1 and pH to achieve is 0,7
Ka1 = c[H3O+] * c[HOA-] / c[H2OA]
c[H3O+] = c[HOA-] (due to the reaction, H3O+ coming from 2 H2O = H3O+ + OH- could be neglected at pH = 0,7)
c[H2OA] in equilibrium = original c[H2OA] - c[H3O+]
original c[H2OA] = c[H3O+] + c[H2OA] in equilibrium
original c[H2OA] = 0,200 + ((0,200 * 0,200) / 0,056)
original c[H2OA] = 0,91 mol/l
check: from formerly 0,91 mol/l H2OA there is 0,20 mol/l H3O+, 0,20 mol/l HOA-, 0,71 mol/l H2OA, Ka1 = 0,20*0,20/0,71 = 0,056 which matches
oxalic acid dihydrate molar mass 126 g/mol
oxalic acid anhydrous molar mass 90 g/mol
0,91*126 = 115 g/l (OA dihydrate)
0,91*90 = 82 g/l (OA anhydrous)
OK enough of theory, now the experiment itself.
10,4 g of oxalic acid dihydrate was dissolved in water and the volume adjusted to 90 ml.
8,9 g of l-carvoxime was refluxed with the solution of oxalic acid for 90 minutes in 1 L flask (it was an overkill, 500 ml flask would be enough).
Then cooled down and 600 ml of water added (overkill, 200 ml would be enough) and l-carvone was distilled out with a steam using simple distillation -
steam generator is not necessary. 200 ml of distillate collected. Upper layer separated in separatory funnel. The yield was further "enhanced" by 5-10
drops by returning the 200 ml bottom layer into the flask and distilling out 50 ml of distillate (stop when the distillate was clear and no new oily
drops on its surface) - no need to mess the product by extracting the bottom layer with an organic solvent.
Yield 4,8 g.
It has nice minty scent, resembles me Wrigley spearmint chewing gum of my childhood.
R-(-)-carvone = L-carvone has minty scent, but S-(+)-carvone = D-carvone which is present in dill seeds or caraway seeds has completely different
scent.
    
[Edited on 17-3-2020 by Fery]
|
|
|