numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Synthesis of substituted azulenes
Azulenes are beautifully colored compounds which are, for the most part, inaccessible to the home chemist. The original routes to unsubstituted
azulene is a nightmare, requiring multiple days and manipulations for a single step. Not to mention that large amounts of pyridine, and other
expensive reagents are needed.
As part of my search to add azulene to my collection I found that pyrylium salts are a convenient precursor. I described how they can be easily made
here, and this is the continuation.
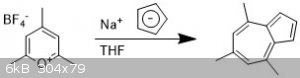
4,6,8-trimethylazulene
I ran this reaction with my dad, to teach him about air-free chemistry. To a small oven-dried schlenk flask with stir bar was added
2,4,6-trimethylpyrylium tetrafluoroborate (500 mg, 1 equiv) and three Ar/vac cycles were done to eliminate atmosphere.

Anhydrous THF (2 ml) was added via a syringe, not everything dissolved. A solution of sodium cyclopentadienide (NaCp) was added dropwise (2M in THF,
1.1 equiv) over 10 min with the appearance of a purple color.

The solution was left to stir for 30 minutes then the solvent was removed to yield a purple red oil.

At this point you can distill it, but I found that running a plug with a few grams of silica and hexanes got rid of most of the impurities. The
remaining semi-crystalline oil can be recrystallized from boiling EtOH to give deep purple prisms. My yield was only 20% but I think this is because I
tried several different things to purify it, each time losing a little. Also the recrystallization is lossy.

The compound is quite pure by NMR, 3 aromatics for the CP ring, 2 for the CH ring, and 9 alkyl protons corresponding to the methyl groups. The NMR was
taken in benzene-d6.
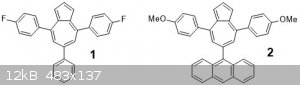
Triarylazulenes
Using two other pyrylium salts I made, the difluoro and the anthracenyl ones, I attempted to use the same conditions to make the respective azulenes
1 and 2.
These reactions were run with my girlfriend while trapped in quarantine. I did this on about 100 mg of each compound, side by side. First dissolved in
THF to give yellow and red solutions.

Then the NaCp solution was added, resulting in drastic color changes. The difluoro compound gave spurts of deep blue on each drop which eventually
faded. The time it took to fade took longer and longer until the color was steady. Sort of like a titration. The anthracenyl compound did the same but
from red to green. Remarkable color changes, really.

After stirring for 30 min, the reaction was quenched with aqueous NH4Cl and extracted with Et2O. We were heartbroken to see the
bright colors turn brown upon quenching, but I'm sure many of you are aware, organo -Li, -Na, -Mg, -K intermediates can be highly colored whereas the
protonated analogs are not. The ether was extracted, dried with MgSO4, and concentrated under reduced pressure to yield a red brown oil.
For 1: TLC showed the presence of a blue species. We elected to use prep-TLC to isolate it, using the crappiest
cardboard-aluminum-foil chamber you've ever seen. But it got the job done. We only got a few (<5) mg of the blue species (top band). The rest was
the orange band which was also isolated to give a red oil.
 
For 2: TLC showed the presence of several nice bright spots, however without other characterization it is impossible to say which.
Both are unpublished compounds, and unfortunately it might be due to synthetic difficulty, rather than lack of attempts. Despite the large amount of
pyrylium salts available, I only found 1-2 papers that use aryl substituted ones to make azulenes. Using alkyl substituted pyrylium salts apparently
works well. I think the blue solid is the desired compound 1, but the low (nonexistant for 2) yields mean scaling up
is not worth it.
Maybe some of you can pipe in, if you have suggestions going forward, or if the orange compound could potentially be the azulene? Hard to tell without
NMR.
Everything was adapted from this orgsynth prep. However as you can tell I heavily modified most of the steps.
Org. Synth. 1964, 44, 94
http://orgsyn.org/demo.aspx?prep=cv5p1088
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Cool! You have quite an impressive lab setup, and you're clearly putting it to excellent use. I'd be curious to hear more about how you set up a
Schlenk system.
|
|
|
numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Indeed it's getting excellent use now. I guess I'm thankful I have something to do at home. Some of my colleagues are going crazy already. I might do
a post on setting up a schlenk system at home if there's sufficient interest. But I feel that technique is far more important in air-free chemistry
than the equipment.
I worked in an organometallic group during my undergrad studies, and every organic group I've been to since has had comparatively bad technique. Sure
they don't need it, but so many people get PhDs now without actually knowing what "air-free" chemistry is. Hoses loosely fitted over joints, far too
much/little grease, wrong type of grease, pump is running on barely any oil that hasn't been replaced in years... the list goes on.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi Numos, more really interesting chemistry! Does this reaction work with unsubstituted pyrylium salts to give unsubstituted azulene?
I would be interested in a thread on a home schlenk system. I have often looked at reactions that require this sort of system but shied away as took
complex.
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Amazing work as always Numos! Interesting synthetic target, I'm fascinated by dyes so I'll be adding this one to my list... Prep TLC is really rare in
the amateur setting so it's nice to see it! Do make a post about your Schlenk system, if only to show off the glassware!
|
|
|
|