| Pages:
1
2
3 |
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
Retort & Stand Questions
Does anyone know of how to use a retort stand? -- I mean is it different from a ring stand?
I'm worried that the cool ring will crack the warm/hot glass.
I am thinking of going with a retort
Still looking for the best link to a lab stand, or in this case a retort stand. -- I'm not sure what the best ones are. united nuclear ones seemed
decent.
also, what about the united nuclear flex clamp? I could clamp it to the edge of my desk and avoid the ringstand all together if it'd support a few
pounds.
thinking of going with the 125mL retort.
Comments?

|
|
|
Sulaiman
International Hazard
    
Posts: 3555
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I like the retro look of a retort
but I can not see any use for one
Why do you want a retort?
If you heat a retort on a ring/retort stand the ring will get heated together with the retort.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
Distillation,
thinking of throwing an ice pack over the spout to aid in condensation.
I think it'll be my only piece of labware this time aside from 6 or so test tubes, and a dropper.
If anyone has a better idea of how to distill solvents without a water cooled condenser, let me know
i.e. - would a stoppered Erlenmeyer with a 25 ft polypropylene tube work better?
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
If you've got 25 feet of tubing you've got a water cooled condenser. Just coil that tubing up in a large can or plastic tub full of water. You'll want
the tubing to exit the bucket/tub/can through a hole in the bottom (seal with silicone or whatnot).
Or if you wait until there's cold weather you can just boil the solvents in an open pan in your room and collect the distillate off the inside of the
windows with a squeegee.
You can also use Cripser technology to condense your distillate:
Drill a hole in the side of your refrigerator just large enough to take the nose of the retort.
Heat the retort on its stand with the retort's nose in the hole in the side of your fridge.
The hot distillate will condense in the cold appliance and the liquid will collect in the vegetable crisper.
Or maybe the meat drawer.
Look, this is cutting edge technology and sometimes there are unexpected surprises.
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
I think i'll be going with a retort.
Perhaps there is some way to connect tubing to the retorts spout -- the only flexible tubing that I Know of that would do this is vinyl, which is
attacked by my solvents.
pvc maybe
I like to use polypropylene
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
thinking I could set the retort's spout into a long cylindrical like vase and submerge that in salt/ice or maybe dry ice / acetone
|
|
|
Sulaiman
International Hazard
    
Posts: 3555
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Using a retort, if you cool the spout with water then the water will probably drip into your product.
Why not consider a cheap ground glass joint 'distillation kit' from China via eBay etc. ?
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
Quote: Originally posted by Sulaiman  | Using a retort, if you cool the spout with water then the water will probably drip into your product.
Why not consider a cheap ground glass joint 'distillation kit' from China via eBay etc. ? |
those require running water, + I like the look of the retort/ Id like to avoid purchasing more stands. Simple is oftentimes better
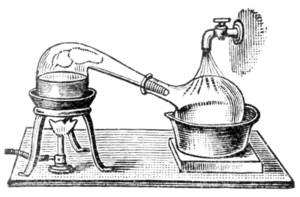
[Edited on 4/23/2020 by Yttrium2]
|
|
|
CharlieA
National Hazard
   
Posts: 645
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
You can put a cheap aquarium pump in a bucket of water to cool a condenser. If you need the condenser to be colder, add ice cubes (or plastic bags of
them) to the bucket.
Distilling acetone in a retort does not seem like a good idea. Perhaps this is why alchemists are a rare breed today.
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
A retort is essentially an air condenser. Useful when you want simplified apperatus and you don't need large rates of heat transfer to achieve
condensation. Also good when there is a reason to avoid water cooling.
There is a member here who routinely uses a retort for distillation of sulfuric acid. Search "boiliing the bat" for details.
I would try to avoid active cooling on the retort itself. And I would try to avoid a metal ring stand unless I could insulate it somehow. (A few
strips of glass fabric to prevent the retort directly touching the metal would be good.) I am thinking the logistics of say 300°C distillations here
and the fact that you want to avoid any thermal stresses as much as possible.
I agree that they look both classic and cool and that might be reason enough to purchase one. It is on my glass fetish list. But realistically, I
don't need one for anything that I am doing.
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
Is there any other way to support my retort?
without using fiberglass strips?
|
|
|
morganbw
National Hazard
   
Posts: 561
Registered: 23-11-2014
Member Is Offline
Mood: No Mood
|
|
This is coming from someone who is using ethanol so please check. It seems that I read a few decades ago that a wire mesh was okay?
|
|
|
Sulaiman
International Hazard
    
Posts: 3555
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
You could use a sand bath for heating and supporting the retort.
+1 on not cooling the arm of the retort,
thermal stress and drips going into your product receiver.
+1 on the bucket of water with aquarium pump,
choose wisely between a.c. line voltage, 12 Vdc, or 5 V USB adapter or power bank.
Also, consider a submersible pump, or a more generally useful external (not submerged) pump.
Finally, consider a cpu cooler type of combination of pump plus radiator with fan(s).
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Chemorg42
Hazard to Others
  
Posts: 103
Registered: 12-11-2019
Member Is Offline
Mood: Concentrated
|
|
Quote: Originally posted by SWIM  | Or if you wait until there's cold weather you can just boil the solvents in an open pan in your room and collect the distillate off the inside of the
windows with a squeegee.
You can also use Cripser technology to condense your distillate:
Drill a hole in the side of your refrigerator just large enough to take the nose of the retort.
Heat the retort on its stand with the retort's nose in the hole in the side of your fridge.
The hot distillate will condense in the cold appliance and the liquid will collect in the vegetable crisper.
Or maybe the meat drawer.
Look, this is cutting edge technology and sometimes there are unexpected surprises.
|
WHAT!
Are these serious suggestions?
Even if they are not, I feel like I need to point out for anyone looking for advice on this thread, do not, repeat, do not do either of these things.
The first is most probably dangerous (not to mention inefficient), while the second violates one of the cardinal rules of hobby chemistry, keep away
from food!
Apologies if this is supposed to be some joke, but I felt I had to respond.
Anyone who is not shocked by quantum theory has not understood a single word. (attributed to Niels Bohr)
I think I can safely say that nobody understands quantum mechanics. (Richard Feynman)
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
@ Chemorg42
Yeah it was a joke. You will get to know SWIM if you stick around. You should have got the CRISPR reference.
|
|
|
Chemorg42
Hazard to Others
  
Posts: 103
Registered: 12-11-2019
Member Is Offline
Mood: Concentrated
|
|
@sum1 Yes, I thought it was probably a joke, but the instructions were just specific enough, and the ideas just out there and crazy enough, that I
thought someone in the world might have meant it seriously.
As a joke, it is really quite good.
Anyone who is not shocked by quantum theory has not understood a single word. (attributed to Niels Bohr)
I think I can safely say that nobody understands quantum mechanics. (Richard Feynman)
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Yeah, sorry about that.
It does sound way too much like actual instructions you'd find somewhere.
It was a foolish way to try to suggest that retorts tend to leak at least some fumes in most set-ups , so don't use one to distill your pyridine.
|
|
|
Chemorg42
Hazard to Others
  
Posts: 103
Registered: 12-11-2019
Member Is Offline
Mood: Concentrated
|
|
Quote: Originally posted by SWIM  | Yeah, sorry about that.
It does sound way too much like actual instructions you'd find somewhere.
It was a foolish way to try to suggest that retorts tend to leak at least some fumes in most set-ups , so don't use one to distill your pyridine.
|
No, it was very funny, well done!
I could just imagine some idiot meth cook seeing that and saying using my fridge, to cook? Ya, sounds like a good idea!
Anyone who is not shocked by quantum theory has not understood a single word. (attributed to Niels Bohr)
I think I can safely say that nobody understands quantum mechanics. (Richard Feynman)
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
All jokes aside, I'll probably go with what is in the depiction I posted
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Hope you're not going to distill solvents with it...
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
Well, not the exact depiction. I'm going to use a electric water boiler as my heat source ( I think) -- which I guess is still a bit of a fire hazard.
I'm not really sure how well my vapors will condense, I think so long as I don't heat it up too fast, it will work nicely.
Even if I were to distill flammable solvents in a retort like the one in the depiction, I am thinking that it wouldn't pose any serious problems if I
did it outside.
|
|
|
Sulaiman
International Hazard
    
Posts: 3555
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Quote: Originally posted by Yttrium2  |
Well, not the exact depiction. I'm going to use a electric water boiler as my heat source ( I think) -- which I guess is still a bit of a fire hazard.
I'm not really sure how well my vapors will condense, I think so long as I don't heat it up too fast, it will work nicely.
Even if I were to distill flammable solvents in a retort like the one in the depiction, I am thinking that it wouldn't pose any serious problems if I
did it outside. |
If you intend to use a water bath to heat the contents of the retort
then the boiling point of the contents must of course be lower than the boiling point of water.
So the temperature will be not much above ambient temperature,
which, combined with the small effective cooling area, will only allow a VERY slow condensation rate.
So slow that you may give up chemistry in despair.
As a rough guide:
if I distill water using a Leibig condenser such as supplied in a cheap Chinese distillation kit,
I can condense about 6 ml per minute 
Using a retort you should plan on much slower rates.
A retort is more suitable for higher boiling point liquids than it is for low b.p. liquids.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
Quote: Originally posted by Sulaiman  | Quote: Originally posted by Yttrium2  |
Well, not the exact depiction. I'm going to use a electric water boiler as my heat source ( I think) -- which I guess is still a bit of a fire hazard.
I'm not really sure how well my vapors will condense, I think so long as I don't heat it up too fast, it will work nicely.
Even if I were to distill flammable solvents in a retort like the one in the depiction, I am thinking that it wouldn't pose any serious problems if I
did it outside. |
If you intend to use a water bath to heat the contents of the retort
then the boiling point of the contents must of course be lower than the boiling point of water.
So the temperature will be not much above ambient temperature,
which, combined with the small effective cooling area, will only allow a VERY slow condensation rate.
So slow that you may give up chemistry in despair.
As a rough guide:
if I distill water using a Leibig condenser such as supplied in a cheap Chinese distillation kit,
I can condense about 6 ml per minute 
Using a retort you should plan on much slower rates.
A retort is more suitable for higher boiling point liquids than it is for low b.p. liquids. |
Even in the depiction there isn't adequate cooling surface area? That is what I plan on doing, not just using the spout of the retort to condense
vapor.
Does anyone have any suggestions as to how to modify the neck of the retort so that it connects to an air cooled condenser/ longer length of tubing?
Lastly, can someone explain, (as I've heard before) - Why higher b.p. compounds condense easier at room temp than the lower b.p. compounds? -- I think
I get it, but a clear explanation might do wonders.
Thanx all
|
|
|
cyanureeves
National Hazard
   
Posts: 737
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
i got lazy trying to dig up condenser,bent arm joints,kleck clips,igloo cooler with built in pump,hoses and flasks so i used my retort the last 4
times. all you need is a pc. of wood with a hole in it where you can slide the cooling arm into.i always use a clamp to hold up the whole retort and i
grab it from the cooling arm right at the base.now thinking it can be done with a pc of wood too really like i stated before.also the last runs i did
were on windy days so i covered the flask in aluminum foil like a bell.it trapped all the heat okay but had better luck even wrapping the cooling
arm.i just stuck the retort arm into a brown jar in an ice bath and hardly any fumes escaped.my retort is not as long as yours and the arm is a bit
thicker probably.its a 500 ml.this summer i will dig out my condenser set up maybe.naw i think i'm done distilling.
|
|
|
zwt2
Harmless

Posts: 17
Registered: 3-9-2019
Member Is Offline
Mood: CHoCoLaTe
|
|
When I was getting started, I bought a retort. Then I used it.
Then I bought a real distillation set.
|
|
|
| Pages:
1
2
3 |