numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Some Iron carbonyl compounds
Just a few iron carbonyl complexes I made for fun. Organoiron chemistry is not so
sensitive and can be carried out at home with limited schlenk equipment. For the most part, metal carbonyls are great precursors to other
organometallic compounds, being a convenient source of M0. Unfortunately, iron pentacarbonyl, along with many other metal carbonyls are not
possible to access unless you have a tank of CO, or some connections to a chemical supplier. That being said, once you have some, the possibilities
are endless. The chemistry in this post is OLD, discovered in the 1920-1950 era, so it's mostly worked out and well documented. It is also important
to note that volatile carbonyls are quite toxic, most of the papers I have seen give a warning as to its toxicity, and if papers in the 1930s are
telling you to be careful.... you'd better be careful!
The compounds in this post are :
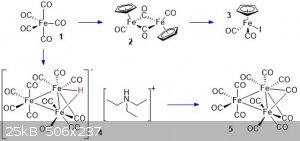
Cyclopentadienyliron dicarbonyl dimer (2)
I believe this compound was previously posted here, I followed the same procedure, but provided some pictures as well. A 250 mL oven dried flask was fitted with a stir bar and water condenser,
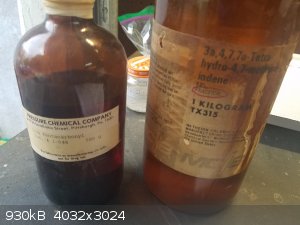
evacuated, and refilled with Ar. Under strong argon flow was quickly poured in dicyclopentadiene (50 mL) and Fe(CO)5 (12.5 mL). The
reaction was vigorously stirred at room temperature for an hour under Ar to remove dissolved oxygen. The reaction was heated to exactly 135 oC, I used
a heating mantle that I cranked up bit by bit until the temperature was stable. Here are the ancient bottles, and a sample of the pentacarbonyl.
Although it is slightly air sensitive, it can be measured out in a graduated cyclinder if you work fast and are in a well-ventilated area.
After heating for 20 h under Ar, the reaction was cooled to RT, hexanes was added (40 mL) and the flask placed in the freezer overnight. The crystals
were broken up, filtered and rinsed with hexanes to yield 2 as a dark purple solid (13 g, 74%). Here is also a rare opportunity to
see my molten fridge...
 
Cyclopentadienyliron dicarbonyl iodide (3)
This reaction can be done in air, without any preparation. Iodine (0.5 g) was dissolved in DCM (25 mL) and added dropwise to a flask of solid, stirred
2 (0.5 g) over the course of 30 min. The solution was stirred for a further 2 hours and a dilute solution of sodium thiosulfate
added. This precipitated a lot of crap, and the entire biphasic mixture was filtered through a celite plug. The organic layer was further washed with
dilute sodium thiosulfate solutions, and with 5% hydrogen peroxide. The solvent was evaporated to dryness, and the crude product purified by a silica
plug (EtOAc/Hex 3:1) to produce a black crystalline solid. The yield of 3 was about 50% due to mechanical losses.
 
Triethylammonium hydrogen undecacarbonyltriferrate (4)
It took me ages to find the structure of 4, it's a rather obscure compound. To a 100 mL flask with a condenser and stir bar is added
distilled water (24 mL) and triethylamine (8.3 mL). Ar was bubbled through the mixture for 5 minutes with stirring to degas the solution and
pentacarbonyl 1 is added (11 mL). The mixture is heated to 80 oC overnight under Ar and then left to cool. The red oil solidifies to
crystals upon cooling, which collected by filtration and rinsed with water several times. The solid is air stable and left to dry in air for several
hours, yielding about 12 g of pure 4 as a dark solid. Note that this compound is actually a salt!
 
Triiron dodecacarbonyl (5)
In a beaker, 4 (2 g) was dissolved in MeOH (5 mL). To this was added 16% aqueous HCl (9 mL) and the mixture stirred vigorously with heating (~70 oC).
There is significant frothing as H2 and possibly CO is liberated. After a while the solution becomes clear with the title compound floating
as a black solid. The solid is filtered, rinsed with water and MeOH and dried to yield about 2 g of 5 as a dark solid. The powder is
really fine and has the potential to be pyrophoric. I had no issues, but something to be wary of.
  
Although 2, 4, 5 are all black solids with slight hues under bright light, their solutions are
highly colored. Here are tiny amounts of the respective pure compounds dissolved in acetone. This dark green is fairly unusual for an Iron0
species.

There are loads more compounds you can make, I chose to make these because 1, 2, 3, and
5 are fantastic jump points to make anything else. For instance 5 can be reacted with many ligands to displace a
single, or multiple CO and make some interesting triiron clusters. 2 is a branching point for "piano stool" type complexes, and
1 has endless uses. I've made a few of these, but I won't spoil everything! 
References:
For 2,3 - Electrochimica Acta, 2018, 283, 27
For 4,5 - Inorganic Synthesis, 1966, 8, 181 (actually a paper by Wilkinson!)
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Nice! I remember making compounds 2 (Fp2) and 3 (FpI) the same way when I was an undergrad (part of a third-year course), but I don't remember what
else we did with them. I think I also made FpCl. (The CpFe(CO)2 moiety is often abbreviated Fp, pronounced "fip". And the word "moiety" doesn't get
enough love outside of organometallic chemistry.)
Oh, yes, compound 2 can be thermally decomposed to give ferrocene, in a lab that made a serious impact on me as a teaching assistant. I had a student
named Sammy. Sammy was one of these entitled assholes who intended to get into med school, and was determined to wring out every last mark with the
least amount of effort. Accent on the least effort.
We had our Fp dimer and I told them how to set up the decomposition- hot plate in the fume hood, Fp2 on a petri dish, upside-down beaker on top of
that so the ferrocene would sublime onto it. I stressed how this gave off not just carbpon monoxide, but also a bunch of other organometallic toxic
crap so it MUST be done in the fume hood. The lab manual told them in 50-point bold font THIS MUST BE DONE IN THE FUME HOOD!
Sammy set it up on the bench, and asked me, "Duh, is it supposed to smoke like that?"
I nearly killed him. I probably should have.
[Edited on 28-4-2020 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Nice work, I love the last picture! The Inorg. Synth. paper says such solutions will deposit iron mirrors if they are left standing in air - have you
gotten one?
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
|