Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
diffusion pump oil diy alternative?
buying a new vacuum diffusion pump is not even an option with my budget of $0, buying it used might be a thing in the US, but in italy there aren't
many amateurs with a diffusion pump for sale, and buying an used pump fron the US would still cost me an arm and a leg with shipping costs and taxes,
so i went the diy route.
i had in mind to build a vacuum diffusion pump for a ehile already, but i didn't have the right materials.
something changed a few days ago, my mother gave me her copper water bottle (stupid thing she bought in india as a healtier alternative to plastic
bottles, coff coff heavy metal), i had a few ideas for this thing, and in the end i went for the vacuum pump.
i pretty much copied the AX-65 from Agilent. i used the copper bottle as the body, and i used sheet metal to make the internal jet assembly. i tested
it under vacuum and it has no leaks yee, now the issue, the working fluid.
usually diffusion pumps use a silicone based oil or a hydrocarbon based oil, they are shit expensive, so nope, i need an alternative.
i tried with WD40 (don't laugh please), i vacuumed 40ml of WD40 to remove absorbed gases and some of the more volatiles compounds, then i heated it on
my hotplate until boiling for a while, to get in the end just the heavier oils.
commercial diffusion pump oils have a working temperature under vacuum of around 200°C, but stupid me can't braze, or weld, so i soldered everything
with normal Pb/Sn solder, i fear that at 200°C the jet assembly will just desolder itself.
anyway back to the WD40, i tried it in my pump after the various treatments, it looked like it was working (i don't have a vacuum gauge so i just use
the look of the plasma inside the vacuum chamber when i apply 50kv), by working i mean it was boiling, and the jets were directing the vapour down (no
condensation in the bell jar), but i noticed condensed oil in the vinil tubing that connects the rotary vane vacuum pump to the diffusion pump, no
bueno.
i tried to light a plasma to check the vacuum level, the pressure was so high that i could not even get an arc, so the oil had a vapour pressure too
high, even at room temperature.
it is taking me a while to clean everything from the WD40, pretty much everything was coated with it inside the pump and bell jar, and the offgassing
is enough that even without oil on the bottom of the pump the vacuum is still crap.
so back to the beginning, what cheap oil i could use?
mineral oil?
  
[Edited on 30-4-2020 by Ubya]
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
monolithic
Hazard to Others
  
Posts: 435
Registered: 5-3-2018
Member Is Offline
Mood: No Mood
|
|
Generic vacuum pump oil. I don't know if it would work, though. Your main concern is vapor pressure. Purpose made diffusion pump oils have very low
vapor pressure which enable the hard vacuum these pumps can produce. Normal vacuum pump oil is 10^-3 to 10^-5 torr vapor pressure at about room
temperature. Diffusion pump oil is closer to 10^-10 torr. https://www.tedpella.com/vacuum_html/Vacuum_Pump_Oil_Propert... https://www.duniway.com/catalog/diffusion-pumps/diffusion-pu...
[Edited on 4-30-2020 by monolithic]
|
|
|
RedDwarf
Hazard to Others
  
Posts: 161
Registered: 16-2-2019
Location: UK (North West)
Member Is Offline
Mood: Variable
|
|
Does it have to be an oil? How about Benzyl Alcohol, or one of the glycols? Thinking even wider although it would mean needing to keep everything at a
slightly raised temperature and probably wouldn't be cheap, would liquid Gallium work?
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by RedDwarf  | | Does it have to be an oil? How about Benzyl Alcohol, or one of the glycols? Thinking even wider although it would mean needing to keep everything at a
slightly raised temperature and probably wouldn't be cheap, would liquid Gallium work? |
the bottom of the pump is heated, the working fluid boils, the vapour gets redirected down and then to the roughing pump through the jets assembly,
the vapour condenses on the cool walls of the pump and trickles back down where the cycle repeats.
synthetic oils are usually silicone based or polyphenyl ethers, gallium would not work, it boils at a much higher temperature than 200°C lol
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
i measured the boiling temperature of my mineral oil and of my vacuum pump oil.
the mineral oil started boiling at around 130°C (measured externally, i could not get my K probe immerded in the oil under vacuum)
the vacuum pump oil, while way more viscous at room temperature than the mineral oil, boils at just 150°C under vacuum.
to take these measurings, i used a test tube and a rubber bung with a piece of tubing through it. the vacuum was applied, and the bottom of the test
tube heated with a gentle small flame. after a few seconds of boiling (when it actually boils, not just gases escaping) i touched the bottom of the
test tube with my K-thermocouple and took a reading. i expect the temperature to be higher than what i measured, but not by much, so i'm still far
from the 180 °C boiling temperature under vacuum.
other suggestions?
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Twospoons
International Hazard
    
Posts: 1282
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
https://en.wikipedia.org/wiki/Brake_fluid ?
If you have drawings of your build I would love to see them, if you dont mind sharing. A diffusion pump is on my wish-list too. I also am in the same
position as you - no local second hand market for diff pumps, and shipping from the US is prohibitively expensive.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
 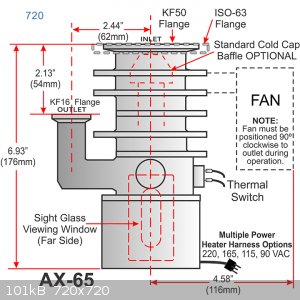 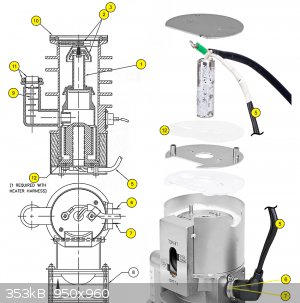  
i just changed the dimensions of the jet assembly to have the same proportions using the copper vessel as reference
i'll also use a heating element inside an aluminium block on the bottom of the pump instead of drilling a hole in the bottom of the copper bottle,
there the temps would be too high for Pb/Sn solder
so i was going for to use another style from another pump

[Edited on 30-4-2020 by Ubya]
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
I think mercury is a pretty common liquid for diffusion pumps, although you'd want a glass diffusion pump for that, and you need like a liter of
mercury. I think the common fluids, at least the one in my edwards diffstack, are chlorinated biphenyls and terphenyls. Keep in mind that you want a
backing pump that can get you to <1 micron, because at the temps these pumps usually run at, having nearly any oxygen is going to decompose your
fluid.
You can buy diffusion pump oil (look for Santovac 5, or MT704) but they are usually very, very expensive. Probably at least $100 per fill. Not sure if
there is any cheaper alternatives, at least if you're trying to get to 1E-4 microns or better... You're best bet might be looking at the patent on how
they make polyphenylethers
[Edited on 4-30-2020 by numos]
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
my roughing pump should be able to get down to 5 Pascals (37 microns), and i have an argon bottle i use to create a low pressure argon atmosphere in
my vacuum system, so i can get rid of oxygen.
mercury is not an option, even if i had a glass diffusion pump.
i know i can buy diffusion pump oil, but it is expensive, and 99% i would need to buy it from overseas, which means high shipping costs and 22% of
taxes on it+customs taxes, that's the main reason i'm trying to find even a not optimal diy solution.
now that you mentioned chlorinated biphenyls and terphenyls oils, i was thinking that maybe i could try chlorinating my mineral oil to give it a
higher boiling point and gravity, it shouldn't be that hard right?
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
trying to chlorinate some mineral oil right now

---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Not sure how easy it will be to chlorinate mineral oils, I think that stuff is mostly alkanes, you might need a light source or something to initiate
radical chlorination. I think chlorinated aromatics are used because they are pretty stable and persistent. Chlorinated alkanes might break down
rapidly under pump temperatures. Also keep in mind you'll want to distill your oil before you use it in your pump, there might be many non-volatile
side products that could polymerize and permanently gunk your pump. The ironic thing - you'll probably need diffusion pump vacuum to distill that
stuff 
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
well i was able to chlorinate it, at least i was able to measure a weight increase of 5%.
a reaction surely happened as the color of the oil went from colorless to yellow and then brown


it got to that darker color after heating to test it's boiling point under vacuum.
i could try distilling it, but i want to make more tests first, like its thermal stability, i'll just run it in my diffusion pump for a few hours, if
i get gunk, i'll clean it, i'm used to it at this point (sad)
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
yobbo II
National Hazard
   
Posts: 709
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Have you tried using the pump yet.
A diffusion pump is on my list..........
There is a terrible cheap looking turbo molecular pump here.
https://www.ebay.co.uk/itm/266046697804?hash=item3df19e714c:...
What is the snag?
I don't suppose you get a 'driver'.
What sort of an apparatus drives the motor does anyone know.
There are alot of pins in the interface plug.
Lots of vac stuff here:
www.belljar.net
Yob
[Edited on 22-12-2022 by yobbo II]
|
|
|
Organikum
resurrected
    
Posts: 2329
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: busy and in love
|
|
Search for "RAVENOL" it is a manufacturer for vacuum pump oils (and other specialities) from Germany, they have an online shop and sell smallish
amounts 1000 ml at industrial prices like 5 to 10 € per liter. They have all variations for all kinds of pumps including diffusion pumps.
Fluorinated oils are of course more expensive.
Or you get Benzylbenzoate bp 330°C+ from S3 (Germany) or laboratoriumsdiscounter.nl or any other place they sell it.
|
|
|
yobbo II
National Hazard
   
Posts: 709
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
There is a read on diffusion pump oils here:
http://www.belljar.net/tBJ_First_Five_Years.pdf
page 1-13
A cheap way to get silicon oil is to distill WD 40. The silicon oil is left behind. (So I believe).
Yob
|
|
|
yobbo II
National Hazard
   
Posts: 709
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
When making a diffusion pump what size holes on the 'chimeneys' do you need?
I cannot see it discussed anywhere. Perhaps the size is not important at all.
The holes are where the boiled oil comes rusing out and is then directed downwards.
Perhaps you boil the oiil at a rate to suit the particular sets of holes.
Yob
|
|
|
yobbo II
National Hazard
   
Posts: 709
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Cheap silicone oil can be obtained here (UK)
https://mistralie.co.uk/search?q=SILICONE+OIL
Cheap diffusion pump on ebay (USA)
https://www.ebay.co.uk/itm/144492421800?hash=item21a46b62a8:... zsAAOSwVFNj9Aj6&amdata=enc%3AAQAHAAAA8Ps13xnPHVPk%2BUnsC93zUfWKVF0QdIQWu0jhTDbkTutO9JVZSBvD61IshKuLzj0m8gw4Y4M0lquorjbpeCVbXYVoo%2FpogMLFBrqbH8a
%2FlGtjCaaTTjpI5pYJGUwxCxwNFlHwxp1FgEWdVknSaebnKmzJc7InrXTTmJWoXcrVMm%2BnxWrftpHq5FqQ09xeoEnHuC4aVSuRTeY0DKSqYRjJjCpUsFgcls2x75i3kVYDwFx7ZEARv70pINcUK
N1abSnYc7ZPnT6bSI9yHB5nFX464M%2B8U9tgsUJDqH3yPBaFCGgiul4Uoxz64FT8eR%2BbpVqwiw%3D%3D%7Ctkp%3ABFBM4rCul9hh zsAAOSwVFNj9Aj6&amdata=enc%3AAQAHAAAA8Ps13xnPHVPk%2BUnsC93zUfWKVF0QdIQWu0jhTDbkTutO9JVZSBvD61IshKuLzj0m8gw4Y4M0lquorjbpeCVbXYVoo%2FpogMLFBrqbH8a
%2FlGtjCaaTTjpI5pYJGUwxCxwNFlHwxp1FgEWdVknSaebnKmzJc7InrXTTmJWoXcrVMm%2BnxWrftpHq5FqQ09xeoEnHuC4aVSuRTeY0DKSqYRjJjCpUsFgcls2x75i3kVYDwFx7ZEARv70pINcUK
N1abSnYc7ZPnT6bSI9yHB5nFX464M%2B8U9tgsUJDqH3yPBaFCGgiul4Uoxz64FT8eR%2BbpVqwiw%3D%3D%7Ctkp%3ABFBM4rCul9hh
Please ignore smiley face. It is an artifact of the machine. I am actually very grumpy :-)
[Edited on 8-3-2023 by yobbo II]
|
|
|