Keras
National Hazard
   
Posts: 769
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Distillation quandary :)
Folks,
I think some of you (at least) are familiar the binary mixture diagram showing that tilted "lentil" shape made up of both the "dew point" curve and
the "vapour" curve, where the horizontal axis represents molar fraction of one component and the vertical axis temperature. This graphic tool is used
to model a distillation operation, and it can be used to predict the final composition of the distillate, provided that the curves are accurate.
Now, my question is this: when you get a mixture to boil, unless you're at an azeotropic point, the vapour will have a different composition than that
of the boiling liquid. Usually, the vapour is richer in most volatile compound, which means that the liquid is going to get enriched in the least
volatile one, which pushes the molar composition of the liquid one way or the other (depending what molar fraction you use in your diagram). In other
words, to track that shift in liquid composition, the boiling point is going to change, usually increasing since the liquid loses its most volatile
fraction. So, how can you end up with a distillate enriched with the most volatile compound since, as the distillation goes on, the temperature rises
and the collected fraction gets richer in the less volatile compound? Also, if you keep the temperature constant, the liquid simply stops boiling.
Just curious, and thanks in advance for any enlightening answer 
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Because I'm doing some ethanol distillations at the moment,
and I use alcoholmeter hydrometers scaled in %ABV (alcohol by volume)
I'll explain using this graph :
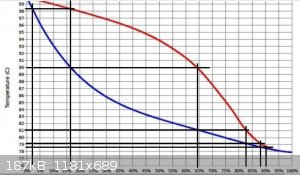
Suppose I start with a 16 %ABV ethanol water mixture doing a 'simple' distillation (no fractionating column)
from the graph, the first few drops of distillate will be about 64 %ABV
as the distillation continues the ethanol concentration in the pot will slowly reduce
as proportionately more ethanol is boiled/evaporated off than water
If the distillation is continued to the point where the ethanol concentration in the pot is reduced to 2 %ABV the distillate will be at 16%
So during the distillation the distillate starts at 64 %ABV and slowly drops to 16 %ABV
If all of the distillate is collected in one container then the average %ABV will be about 40
If the distillate was collected in several small 'cuts' then the first cut may be about 60 %ABV, the next about 50 %ABV, then 40 %ABV, 30 %ABV etc.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Keras
National Hazard
   
Posts: 769
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by Sulaiman  |
Because I'm doing some ethanol distillations at the moment,
and I use alcoholmeter hydrometers scaled in %ABV (alcohol by volume) […]
From the graph, the first few drops of distillate will be about 64 %ABV
|
Agreed.
Quote: Originally posted by Sulaiman  |
as the distillation continues the ethanol concentration in the pot will slowly reduce as proportionately more ethanol is boiled/evaporated off than
water. If the distillation is continued to the point where the ethanol concentration in the pot is reduced to 2 %ABV the distillate will be at 16%. So
during the distillation the distillate starts at 64 %ABV and slowly drops to 16 %ABV.
|
Of course, if you distil ALL your starting mixture, you end up with the same mixture in the receiving flask, so nothing is gained. The inevitable
conclusion is that you have to accept a (hopefully not that big) loss of ethanol to be able to concentrate it in good conditions. You can never
recover all of it in concentrated form.
[Edited on 1-5-2020 by Keras]
|
|
|
Texium
|
Thread Moved
1-5-2020 at 05:17 |
Keras
National Hazard
   
Posts: 769
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Ok, this time it worked. I just had to make up with the losses, and I stopped the distillation each time when a third of the mixture had distilled
out. I did three consecutive runs and got something concentrated enough to be ignited by a match. Thanks a bunch!
|
|
|
|