| Pages:
1
2 |
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by AvBaeyer  | Ubya,
Really a nice piece of work! It shows what can be done when experiments are really done and done well. I was a sceptic but you have shown otherwise.
Congratulations.
AvB |
 thanks, i really appreciate it thanks, i really appreciate it
Quote: Originally posted by Housane  | | Ubya, amazing work, what glassware is in those pictures, i really want to try this as i have a load of desicant? |
for that test I used a normal 5ml glass pipette,
[img]https://elenxs4.com/getImage.php?p=1234596&sku=JJ_JJ17210-00-X1.jpg[/img]
(i bought it from ebay).
i tried writing a page about it https://eclecticsclub.wordpress.com/2020/06/02/kitty-litter-...
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Housane
Hazard to Others
  
Posts: 127
Registered: 3-9-2018
Location: Worcester, England
Member Is Offline
Mood: Let’s make
|
|
Wonderful thanks, what do you think this can separate, everything a commercial system could?
[Edited on 6-6-2020 by Housane]
Green QD's so far
Feel free to correct grammar or incorect knknowledge. We are all learning.
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by Housane  | Wonderful thanks, what do you think this can separate, everything a commercial system could?
[Edited on 6-6-2020 by Housane] |
well of course the quality of the silica is not the same as the one used in labs, even if you can get a small range of particle sizes, i bet there are
still big differences in pore sizes.
This silica can be surely used for separating compounds with large retenction factors, so no it's not the same as the commercial product, but it could
make life way easier for a few kinds of separations. for example if you wanted to separate carotenoids from the chlorophylls without using a
chromatography setup, it would not be easy.
for example my main reason for trying this is that i wanted to remove the colored compounds from hot pepper extracts, and get a pure clear oil instead
of the reddish oil you get by extracting capsaicin from hot peppers
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Housane
Hazard to Others
  
Posts: 127
Registered: 3-9-2018
Location: Worcester, England
Member Is Offline
Mood: Let’s make
|
|
Thanks Ubya, that makes lots of sense, i am building my own now
Green QD's so far
Feel free to correct grammar or incorect knknowledge. We are all learning.
|
|
|
Mateo_swe
National Hazard
   
Posts: 505
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
That experiment seems to have worked very well, nice work.
Do you plan on making some runs with the kitty litter in your 50cm column?
This would be the new KLLC technique, or maybe UKLLC for Ubyas KLLC.
Im interested in what can be done with this and hope you continue the experiments.
Hopefully one can use this to separate compounds from different reactions, that would be really nice.
Keep up the good work.
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by Mateo_swe  | That experiment seems to have worked very well, nice work.
Do you plan on making some runs with the kitty litter in your 50cm column?
This would be the new KLLC technique, or maybe UKLLC for Ubyas KLLC.
Im interested in what can be done with this and hope you continue the experiments.
Hopefully one can use this to separate compounds from different reactions, that would be really nice.
Keep up the good work. |
I'll surely use that in my CV
yeah, i'll do a few runs on my 50cm column, i had the idea of using kitty litter because if i were to use commercial silica, each run could cost me
5-10 euros.
the next step in my research is simply getting a smaller range of particle sizes. I bought a 120 mesh screen (from china it will take 1 month at least
to arrive here), so i can get only particles between 125 and 150 micrometers, hopefully improving separation.
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Mateo_swe
National Hazard
   
Posts: 505
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
So you think 100 mesh had too much resistance?
I read that the first test was particle size smaller than and up to 100.
If one were to try this what would be a suitable size of column?
I see 300mm tall columns in diameters 20 to 45mm is sold pretty cheap.
Some have a porous filter in the bottom like the one in some funnels and some have a PTFE Stopcock to regulate the flow.
Also you used hexane and acetone, is any other solvents good for this that isnt very expensive?
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by Mateo_swe  | So you think 100 mesh had too much resistance?
I read that the first test was particle size smaller than and up to 100.
If one were to try this what would be a suitable size of column?
I see 300mm tall columns in diameters 20 to 45mm is sold pretty cheap.
Some have a porous filter in the bottom like the one in some funnels and some have a PTFE Stopcock to regulate the flow.
Also you used hexane and acetone, is any other solvents good for this that isnt very expensive? |
when i did the test in that pipette, i used a crappy packing technique, also my powder right now is "everything smaller than 100 mesh" so a big part
of it could have been much finer.
the size if the column depends on how much compound you need to purify,
about the solvents, you just need polar and apolar solvents (for simple purifications), use whatever is common to you, (if you use ethanol remember
that it must be water free), you can use petrolium ether as an apolar solvent, there are many tables showing you the polarity of every solvent/solvent
mixture
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Mateo_swe
National Hazard
   
Posts: 505
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
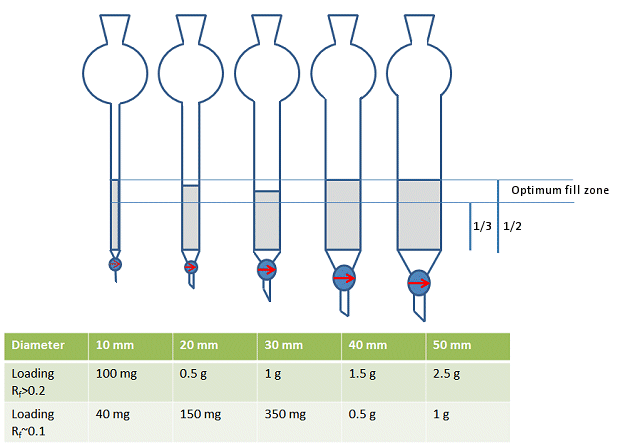
OK, so one only uses half to 1/3 of the columns length for the SiO2.
I thought the whole column was used and a chromatography flask was put on top to hold the solvent. I clearly must read up on chromatography.
If say 10g was to be purified, it would need a very big column.
I know very big columns is used to separate compounds in industry, are they using the same principle just upscaled?
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by Mateo_swe  | OK, so one only uses half to 1/3 of the columns length for the SiO2.
I thought the whole column was used and a chromatography flask was put on top to hold the solvent. I clearly must read up on chromatography.
If say 10g was to be purified, it would need a very big column.
I know very big columns is used to separate compounds in industry, are they using the same principle just upscaled? |
https://www.youtube.com/watch?v=jl__He482G4&t=207s
they don't look like lab columns, but yeah they use the same principles
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by Ubya  |
for example my main reason for trying this is that i wanted to remove the colored compounds from hot pepper extracts, and get a pure clear oil instead
of the reddish oil you get by extracting capsaicin from hot peppers |
Please let us know how this goes! I have a lot of Carolina Reapers in methanol standing for ages now. I once got a nice beige powder after some kind
of crystallization (I really don't remember what I did...), but I couldn't reproduce and got lots of red oil afterwards.
The pure capsaicins are solid by the way.
|
|
|
| Pages:
1
2 |