goldberg
Hazard to Self
 
Posts: 90
Registered: 28-4-2018
Member Is Offline
Mood: No Mood
|
|
Alternatives to Friedl-Crafts reaction
I'm curious if there is other way to alkilate/acylate aromatic ring beside Friedl-Crafts reaction.
Especially i'm interested in preparation of benzyl ketones.
Major drawback of Friedl-Crafts acylation is requirement for large amount of AlCl3 that is destroyed after reaction and acid chloride/anhydrite that
is ususaly difficult/dangerous to prepare.
I read about Quelet reaction but for some reason it is very rarely mentioned at all.
There is also Nencki variant of Friedl-Crafts reaction but i read that yields are poor.
Grigrard reaction requires strict conditions so i'm not counting it here.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Pyrolysis of calcium or iron salts of benzoic acid and the desired carboxylic acid chain.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Aryl bromides can be zincated in acetonitrile with cobalt bromide as catalyst. This is a bit nicer than a Grignard because acetonitrile is less
dangerous than ether. The arylzinc reagents are a little more robust as well.
https://pubs.acs.org/doi/abs/10.1021/ja0289494 (attached)
Applicable electrophiles are aldehydes, acyl halides, CO2 and possibly epoxides. Allyl acetates have been reported as well, which is kind of
surprising:
https://pubs.acs.org/doi/abs/10.1021/ol0340641
Transmetallation of arylzinc reagents with CuCN*2LiCl might allow coupling with other electrophiles as well:
https://www.sciencedirect.com/science/article/abs/pii/S00404...
Attachment: fillon2003.pdf (61kB)
This file has been downloaded 337 times
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
outer_limits
Hazard to Others
  
Posts: 139
Registered: 3-3-2020
Member Is Offline
Mood: hybridized
|
|
You can perform Friedel-Crafts acylation without acyl chloride or anhydride using carboxylic acid and P2O5 with Al2O3.
IIRC Chemplayer uploaded video in which he did it using caboxylic acid, P2O5 and AlCl3, but his yield was quite low.
Attachment: hajipour2009.pdf (313kB)
This file has been downloaded 360 times
[Edited on 17-5-2020 by outer_limits]
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Plain polyphosphoric acid, made from P2O5 and 85% H3PO4, works to make ketones via FC-acylations, no need for aluminium salts.
|
|
|
Johnny Windchimes
Hazard to Self
 
Posts: 61
Registered: 28-5-2019
Member Is Offline
Mood: Sorry, it's my chimes~!
|
|
Caveat Emptor on Iranian chemistry IMO, but give this a looksie:
Uses Zinc Oxide instead of AlCl3 or fancy pants stuff.
Attachment: sarvari2004 - friedel with ZnO - Copy.pdf (73kB)
This file has been downloaded 449 times
~Incredibly profound and/or wise quote goes here~
|
|
|
goldberg
Hazard to Self
 
Posts: 90
Registered: 28-4-2018
Member Is Offline
Mood: No Mood
|
|
Thanks. I like idea of using zinc oxide as catalyst.
If we are talking about Friedl-Crafts acylation is there any healthier alternative to chlorinated solvents for this reaction?
Le'ts assume that aromatic compound in this reaction is solid so it can not be used as solvent.
It is possible to use nitrobenzene or similar compound that has deactivating group attached to aromatic ring.
How about esters? Say ethyl acetate for example? Since esters can be synthesised from acid chlorides then they should not react with them, right?
Has anybody practical experience with Barbier reaction(variant using zinc)? It seems to be of rather limited scope.
|
|
|
Johnny Windchimes
Hazard to Self
 
Posts: 61
Registered: 28-5-2019
Member Is Offline
Mood: Sorry, it's my chimes~!
|
|
Quote: Originally posted by goldberg  | Thanks. I like idea of using zinc oxide as catalyst.
If we are talking about Friedl-Crafts acylation is there any healthier alternative to chlorinated solvents for this reaction? |
Sarvari uses NO solvent for a few of the reactions, and of course, these solvents:
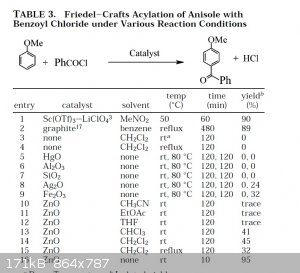
~Incredibly profound and/or wise quote goes here~
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
The method using polyphosphoric acid is also often done without a solvent, and can be greatly improved in terms of yield by microwave conditions.
One of its advantages is, that it works even on rather sensitive substrates, for example benzodioxol or other methoxylated benzenes.
It is even used in Pihkal: https://erowid.org/library/books_online/pihkal/pihkal061.sht...
|
|
|
goldberg
Hazard to Self
 
Posts: 90
Registered: 28-4-2018
Member Is Offline
Mood: No Mood
|
|
I'm curious why acylation with ZnO does not occur in ethyl acetate.
Route with polyphosphoric looks nice.
How about electrochemical acylation? i found one paper about electrochemical version of Frield-Crats(didn't have this paper at hand right now) but i'm
curious about other possibilities.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I'm pretty sure OP said "benzyl" ketones not "aryl" ketones so I'm not sure all these acylations are on the right track.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Oh crap.
I just read the title and the others suggestion well enough, and thought that FC acylations were in order because the others suggested acylation
reactions, and not FC alkylations, shame to admit 
| Quote: |
Route with polyphosphoric looks nice. |
This is probably one of the mildest and most versatile method for the friedel-crafts acylation... however, it does not work for the
FC-alkylation.
So, benzyl ketones aren't possible to make with it.
If you want to make benzyl ketones with the FC, you have to use the FC-alkylation and not the acylation, because that will result in phenyl ketones
only.
|
|
|
Tellurium
Hazard to Self
 
Posts: 84
Registered: 12-7-2017
Location: Group 16, Chalcogen City
Member Is Offline
Mood: smelly
|
|
What could also be an option would be radical alkylation using Manganese(III) acetate. Chemplayer also tried that route out for the alkylation of
benzene with acetone, but I think they weren't successful.
In some publications I saw the use of a alkyl halogenide when doing a radical alkylation with manganese(III) acetate, so maybe thats the key to
success?
https://www.researchgate.net/publication/228630059_Manganese...
I never tried that out personaly, but if that works it would be great, because it really sucks, that in FC Acylations the AlCl3 is used up and doesn't
just works as catalyst, like in FC Alkylations, so you need to use it in molar excess.
|
|
|
goldberg
Hazard to Self
 
Posts: 90
Registered: 28-4-2018
Member Is Offline
Mood: No Mood
|
|
Sorry, that was my mistake. I'm intrested in typical acylation reaction just mistaken name of product.
In parallel i'm intrested in alkilation reaction in general. Acylation + deoxygenation of product is nice because there is no problem with
overalkilation and rearrangement. But maybe there is some one-step alternative?
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
What do you mean with deoxygenation?
Do you mean you want to get to, like from the FC-acylation to for example propiophenone, to get even further in one-pot to propylbenzene in that case?
So you actually want to use the acylation only because it does not overalkylate, but actually you don't want to keep the carbonyl and desire an alkane
chain as your product without it instead?
I don't know if you can do this in one pot, but the clemmensen reduction was used in the link I gave above, from Pihkal, where polyphosphoric acid was
the catalyst and the resulting substituted amylophenone was then reduced to the amyl chain just as you want it.
If I understand you right, that is.
|
|
|
goldberg
Hazard to Self
 
Posts: 90
Registered: 28-4-2018
Member Is Offline
Mood: No Mood
|
|
Yes i'm thinking about conversion like You said benzene -> propiophenone -> propylbenzene.
Is it possible to use acid salt with polyphosphoric acid route instead of free acid? Say sodium acetate instead of glacial acetic acid. Free acid
should be generated in situ if i'm right.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
I guess so, should be doable?
|
|
|
dextro88
Hazard to Self
 
Posts: 60
Registered: 20-10-2018
Location: In the lab
Member Is Offline
Mood: loading...
|
|
this procedure is well described in clandestine literature, you can reduce the ketone with borohydride and oxidize to propenylbenzene.
otherwise im very intrested to FC-alkylation alternatives, did anyone have done some experiments to englighten us.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Quote: Originally posted by goldberg  | Yes i'm thinking about conversion like You said benzene -> propiophenone -> propylbenzene.
Is it possible to use acid salt with polyphosphoric acid route instead of free acid? Say sodium acetate instead of glacial acetic acid. Free acid
should be generated in situ if i'm right. |
IF it was possible you would probably need to double the amount of polyphosphoric.
If it's being consumed generating the gaa it's not friedel crafting like it should be and we don't know if the sodium salts inhibit the reaction. It
just seems unnecessary and complicated for no reason.
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Quote: Originally posted by goldberg  | Yes i'm thinking about conversion like You said benzene -> propiophenone -> propylbenzene.
Is it possible to use acid salt with polyphosphoric acid route instead of free acid? Say sodium acetate instead of glacial acetic acid. Free acid
should be generated in situ if i'm right. |
You can do a Friedel-Crafts using benzene, FeCl3 as a catalizer and propionyl chloride that's easily sinthesized from propionic acid and
TCCA/triphenyl phosphine couple (see here: http://www.sciencemadness.org/talk/viewthread.php?tid=80658&...) or propionic acid, cyanuric chloride in acetone and TEA (see here: http://www.sciencemadness.org/talk/viewthread.php?tid=80658&...).
Propiophenone can be reduced to 1-phenyl 1-propanol and Dehydrating the alcohol you get propenyl benzene, following the directions at the paper
attached below, taken from Rhodium pages. Propenyl benzene with peracids gives P2P, you know.
You can also start from styrene and react that with peracetic acid and acetic anhydride to get propiophenone, 1-phenyl 1-propanol and styrene oxide,
like said at the Patent I'm attaching too:
Attachment: styrene to phenyl 1-propanol, propiophenone & styrene oxide.pdf (386kB)
This file has been downloaded 341 times
Attachment: propiophenone, phenyl 1 propanol and propenyl benzene.doc (35kB)
This file has been downloaded 308 times
|
|
|
goldberg
Hazard to Self
 
Posts: 90
Registered: 28-4-2018
Member Is Offline
Mood: No Mood
|
|
dextro88: You meant rather alcohol elimination to yield alkene, not oxidation, right?
Since i'm not interested in any illegal/psychoactive compounds i didn't checked clandestine literature, but if You have any scientificly valuable
references i will be gracefull for them.
@Chemi Pharma: Thank You i will have a look at this papers . TCCA is widely avaiable but i didn't found good source of cyanuirc chloride. How about
using TCCA and DMF? DMF is used as catalyst for preparation of acetic chlorides from oxalyl chloride. Making oxalyl chloride from glyoxal and then
desired acetic chloride from oxalyl chloride would be quite labor instensive...
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Quote: Originally posted by goldberg  | dextro88: You meant rather alcohol elimination to yield alkene, not oxidation, right?
Since i'm not interested in any illegal/psychoactive compounds i didn't checked clandestine literature, but if You have any scientificly valuable
references i will be gracefull for them.
@Chemi Pharma: Thank You i will have a look at this papers . TCCA is widely avaiable but i didn't found good source of cyanuirc chloride. How about
using TCCA and DMF? DMF is used as catalyst for preparation of acetic chlorides from oxalyl chloride. Making oxalyl chloride from glyoxal and then
desired acetic chloride from oxalyl chloride would be quite labor instensive... |
@Goldberg, you don't need to use both reagents to make acid chlorides. Or you use TCCA method or you use Cianuric chloride method. The links I've
given in my last post, one is for TCCA with Triphenyl phosphine method, given originally by @AVBaeyer and the other is about a method I achieve with
researching and tell about Cyanuric chloride in acetone with Triethylamine (TEA). If for you is more OTC to purchase TCCA, purchase too some triphenyl
phosphine and propionic acid and make your own propionyl chloride at home lab easily.
TCCA or cyanuric chloride with DMF as far I know is used to produce alkyl chlorides from aliphatic alcohols. I have a paper about that posted
somewhere here in the forum. I don't know about any route that makes acetyl chloride from oxalyl chloride. If you know any, post the paper here
please.
|
|
|
dextro88
Hazard to Self
 
Posts: 60
Registered: 20-10-2018
Location: In the lab
Member Is Offline
Mood: loading...
|
|
yes in some clandestine papers they use KMNO4, its why i said oxidization but when i writed it i was a bit confused behind the mechanism, as it
clearly states in the paper its dehydration its my wrong, look at this reff :
https://www.designer-drug.com/pte/12.162.180.114/dcd/chemist...
|
|
|