Bromolone
Harmless

Posts: 20
Registered: 16-9-2019
Member Is Offline
Mood: Aldehydic
|
|
Isolation and Synthesis: Benzyne?
Greetings!
Here's another amateur chemist, hailing from India. I was lurking around the forum as a Guest until the recent one-day forum downtime when I decided
to register.
Well, I do have some experience handling corrosive acids, bases, and organics, but I shall be synthesising most of the acids as they're not available
OTC.
[This question might seem stupid]
Benzyne is a highly useful synthetic intermediate for several reactions and is often produced in-situ due to its instability. The HDDA (hexadehydro
Diels-Alder) reaction, dehalogenation of aryl halides, etc are preferred methods for generating arynes but are not 'readily' available to the amateur
chemist (as far as I could Google). Most reactions involving arynes call for their synthesis as an intermediate in the reaction. How about isolating
it for a short-term?
Diphenylene forms by the dimerization of benzyne, in good yields. Is there a way to 'trap arynes' in an isolable manner - avoiding the dimerization -
in a bottle?
Note:
I have just ventured into amateur chemistry and do not possess any equipment required for any sort of distillations (coming soon) and haven't
performed any yet. My first projects in distillation would be crude ethanol and trichloromethane. I have no hands-on experience in organic syntheses
at the moment, but at 18 I find it better to first start off with simple inorganic experiments with safety equipment. 
I remember how one day my entire class was working in the lab without gloves with nitric acid in school. 
Also, I do deem ScienceMadness as one of my life-long compaions in my journey ahead.
[Edited on 1-6-2020 by Bromolone]
Good documentation is key to good chemistry and software alike.
|
|
|
12thealchemist
Hazard to Others
  
Posts: 181
Registered: 1-1-2014
Location: The Isle of Albion
Member Is Offline
Mood: Rare and Earthy
|
|
Welcome to Sciencemadness! And a very interesting question to start with! Unfortunately, the short answer is that it's not possible in the sense you
mean. This is because the alkyne is so very strained. There are resonance forms can be drawn:
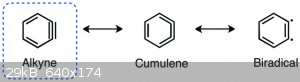
As you can see, the alkyne and cumulene (which like 180° angles) are bent to 60°, immensely increasing reactivity. Radicals, as you are probably
aware, are extremely reactive and rarely stable, and the diradical form also shown helps explain benzyne's extreme reactivity.
The reason why benzyne is never isolated is that it is impossible to do so, except under very carefully controlled conditions. The classic for this is
isolation within a matrix. The compound to be studied is prepared in the gas phase, and then frozen in argon or some inert gas at very low
temperatures. The low temperature and physical seperation is what enables the compound's isolation.
Another common method (a good example being carbenes) is to coordinate the compound in question to a metal. Somewhat unusual since it uses uranium,
but https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.2013039... is an illustration. For carbenes, consider Grubb's catalyst.
Finally, encapsulation within a macromolecule is the last option I'm aware of that is commonly used. Again, this is physical separation from other reactive
species, enabling "isolation".
As may be clear, most if not all of these options are extremely difficult for the amateur to achieve, and actually lie at the frontier of science.
But, having said that, the same used to be said of carbenes - species of the type R2C: with a lone pair. The easiest for the amateur to
prepare would be dichlorocarbene, :CCl2. However, these are short-lived species normally, but then persistent carbenes were discovered, of
which a large category are N-heterocyclic carbenes (NHCs). Wikipedia gives a good enough background to this area.
|
|
|
Sulaiman
International Hazard
    
Posts: 3555
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Welcome Bromolone
Concentrated nitric acid is best handled carefully without cheap gloves,
fuming nitric acid ignites nitrile and latex gloves.
e.g. https://www.youtube.com/watch?v=aBVdGGml6bU
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Bromolone
Harmless

Posts: 20
Registered: 16-9-2019
Member Is Offline
Mood: Aldehydic
|
|
| Quote: |
fuming nitric acid ignites nitrile and latex gloves
|
Thanks, Sulaiman!
| Quote: |
most if not all of these options are extremely difficult for the amateur to achieve
|
Thanks for the references, 12thealchemist! Benzyne is indeed out of our reach to be stored in a bottle 
(Well, the preprative difficulty of other interesting stuff - say, salts of the pyrylium cation - is far less than benzyne isolation and seems like a
good idea for some advanced organic synthesis!)
[Edited on 1-6-2020 by Bromolone]
Good documentation is key to good chemistry and software alike.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
The smallest possible ring of alternating triple bonds and single bonds is C18. https://en.wikipedia.org/wiki/Cyclo(18)carbon
|
|
|
Bromolone
Harmless

Posts: 20
Registered: 16-9-2019
Member Is Offline
Mood: Aldehydic
|
|
Thanks, Cou.
Cyclooctyne is the smallest cycloalkyne that is isolable and can be stored. Cyclopentyne and some other lower members have been reported as complexes
with certain metals.
https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.1989129...
[Edited on 3-6-2020 by Bromolone]
Good documentation is key to good chemistry and software alike.
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
If you're interested in performing a Diels-Alder reaction using benzyne, it is actually possible in spite of its instability. When anthranilic acid
undergoes diazotization and decarboxylation in the presence of a suitable diene, the benzyne that is momentarily formed can be trapped: https://www.chemtube3d.com/dabenzyne_formation/#:~:text=Benz...
It's a really neat reaction!
Anthranilic acid itself is a good intermediate level synthesis once you become more experienced with organic chemistry. Here's my write-up of it: https://texiumchem.com/2017/03/22/preparation-of-anthranilic... There's links to Chemplayer's excellent video demonstrations from there as well.
|
|
|
Bromolone
Harmless

Posts: 20
Registered: 16-9-2019
Member Is Offline
Mood: Aldehydic
|
|
Thanks, Texium!
I had already planned to follow your preparation of anthranilic acid after nitrobenzene and aniline  . Happy to see you back on your blog. . Happy to see you back on your blog.
And yes, I do need to get more experienced in organic chemistry as I'm yet to prepare phthalimide from the not-so-easily-available-here phthalic
anhydride.
The Chemplayer video links on your site only show up the videos as nonexistent on YouTube; all are available here tho:
https://archive.org/download/ChemPlayer 
Good documentation is key to good chemistry and software alike.
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
Ah, right. Thanks for reminding me about ChemPlayer. I should change the links to lead to their BitChute uploads instead.
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
I did my undergrad work on benzyne chemistry (specifically, the HDDA reaction). PM me if you'd like to discuss this more.
| Quote: | | How about isolating it for a short-term? Is there a way to 'trap arynes' in an isolable manner - avoiding the dimerization - in a bottle?
|
Unfortunately no; they're extremely reactive as electrophiles and cycloaddition partners. If they can't find anything else to react with, they'll even
rip H2 off of solvent and hydrogenate themselves to a stable arene. Any isolation would have to be in the gas phase, or in a matrix of frozen argon.
|
|
|
Bromolone
Harmless

Posts: 20
Registered: 16-9-2019
Member Is Offline
Mood: Aldehydic
|
|
Thanks, Metacelsus; I had already read your post on your thesis before starting this topic!
Solomons' Organic Chemistry suggests Texium's anthranilic acid method for temporary benzyne trapping, and I have got quite a lot of reagent and
apparatus acquisition to do before I start off (shipping delays, basically). I will contact you once I synthesize anthranilic acid so I can later
diazotize it and work on some Diels-Alder reactions.
If you might have some other methods in mind (I'm yet to read the doc you uploaded in the thread), I'll discuss them then! 
[Edited on 8-6-2020 by Bromolone]
Good documentation is key to good chemistry and software alike.
|
|
|
Methyl.Magic
Hazard to Others
  
Posts: 139
Registered: 14-5-2007
Member Is Offline
Mood: No Mood
|
|
Benzyne is my field of research. I prepare benzocyclobutene with it by trapping the aryne with an alkene.
The easiest way of generating benzyne is refluxing bromobenzene with NaNH2 (strong base). Personally I prepare them from the fluorobenzene, then
deprotonate the alpha acidic proton of the fluorobenzene with BuLi, then on heating LiF is realease along with the aryne 
|
|
|
Bromolone
Harmless

Posts: 20
Registered: 16-9-2019
Member Is Offline
Mood: Aldehydic
|
|
Thanks, Methyl.Magic.
Apologies for not seeing your post soon enough. I'm currently stuck in the transition between high school and uni and I've got several exams coming
up; I can't spare much time for the forum nowadays.
Well, strong bases such as NaNH2 are pretty expensive here OTC and it'll take me long before I start off preparing it in the lab. The anthranilic acid
route seems to be the most convenient synthesis for now.
Note:
The reagents I had ordered have now arrived (lab work to be continued on weekends) and I will return to the forum (in August) with a series of
syntheses of organics I've been waiting to do since long (Trimesaldehyde et al).
Good documentation is key to good chemistry and software alike.
|
|
|
Methyl.Magic
Hazard to Others
  
Posts: 139
Registered: 14-5-2007
Member Is Offline
Mood: No Mood
|
|
NaNH2 expensive ? really ? This is one of the cheapest strong base...
What is your substrate ? maybe I can help you...
|
|
|
Bromolone
Harmless

Posts: 20
Registered: 16-9-2019
Member Is Offline
Mood: Aldehydic
|
|
@Methyl.Magic: Sodamide is indeed expensive in here - about 14 USD for a few grams. Chemicals that one might find easily in other parts of the world -
say acetic anhydride - are banned for individual use by our Narcotics Department and their purchase requires tons of paperwork.
The substrate is the classic for DA reactions: Furan.
And I'm back! (Exams postponed to September  ) )
I've been fairly successful in preparing elementary, essential reagents for several experiments and org-chem workups. Benzyne is far from now but I'm
now working on mesitylene and other symmetric aromatics. A thread shall follow this up.
Good documentation is key to good chemistry and software alike.
|
|
|