reactofurnace
Hazard to Self
 
Posts: 76
Registered: 17-7-2015
Member Is Offline
Mood: Volatile
|
|
Question help
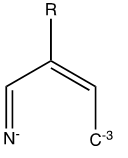
Heyy. Does anyone have an idea what this ligand is called? Can it form any tetrahedral coordination compounds with Fe2+, Ni2+, Cr2+, Co2+, Mn2+ or
Fe3+.
Any help and/or explanation would be of great help 
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
it's probably called Unstable
tha carbon with only a single bond and 3 negative charges on it, i don't think it could even exist in normal conditions
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
reactofurnace
Hazard to Self
 
Posts: 76
Registered: 17-7-2015
Member Is Offline
Mood: Volatile
|
|
Quote: Originally posted by Ubya  | it's probably called Unstable
tha carbon with only a single bond and 3 negative charges on it, i don't think it could even exist in normal conditions |
I thought the same thing. Perhaps if the carbon had 3 hydrogens attached. Would it result in a tetrahedral geometry then?
|
|
|
ThoughtsIControl
Hazard to Self
 
Posts: 50
Registered: 13-10-2019
Location: Proxima Centauri
Member Is Offline
Mood: Yin over yang
|
|
I haven't officially taken organic chemistry in college yet, but I've watched a ton of youtube videos hahaha
If the carbon was arranged in a tetrahedral geometry with the 3 hydrogens instead of a 3- charge and a covalent bond to the carbon. Then I would think
that this would still be unstable, right? The nitrogen is still negative with a pi bond on it. Won't the molecule try to rearrange so that the carbon
donates hydrogen to the nitrogen to rid the charge? The pi bonds would also rearrange I believe.
Basically I am saying that even if there were 3 hydrogens attached to the carbon instead of a 3- charge --> then a rearrangement will occur in
order to reach stability.
|
|
|
CharlieA
National Hazard
   
Posts: 645
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
Whether the C has 3 hydrogens attached or 3 unshared pairs, it can still be sp3 hybridized and therefore has tetrahedral geometry. Of more interest to
me because I guess I don't understand the structure, what is the configuration of electrons in the outer shell of the Nitrogen atom? To have a charge
of negative 1, the N atom should have 6 electrons in its outer shell, but I count only 5 (maybe): 2 shared electrons + an unshared pair(?) + the -1
charge.
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Crotyl imine tetra anion, the conjugate acid is a compound of the class of a,b-unsaturated imines. The dianion might exist, but I don't know if
anything is known on it forming metal complexes.
[Edited on 10-6-2020 by Sigmatropic]
|
|
|