| Pages:
1
2 |
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Take a melting point.
Try TLC.
We are amateurs but these methods are usable even for us.
|
|
|
myr
Harmless

Posts: 48
Registered: 18-7-2018
Member Is Offline
|
|
Quote: Originally posted by Boffis  | @michaljemco; Thankyou for posting details of your prep.
@myr; how different is this reaction to the preparation of N-Phenylanthranilic acid (See Vogel, Practical Organic Chemistry, 3rd, page 991) from
aniline and o-chlorobenzoic acid? Except that the preparation in Vogel uses a copper oxide catalyst? Would a copper or copper oxide catalyst help in
this reaction? |
That is the Ullmann reaction, or what is called an Ullmann-type reaction, which does require a Cu catalyst. It proceeds through an aryl copper
intermediate which is pretty neat.
https://www.organic-chemistry.org/namedreactions/ullmann-rea...
Quote: Originally posted by michalJenco  | This also makes me wonder about what I actually made when I did this procedure with phenol and 2- and 4- chlorobenzoic acids, where the products after
Fischer esterification with methanol were solids with amazing smells. Methyl 2-chlorobenzoate is a liquid so I didn't make that. I also don't think a
2-chlorobenzoate ester would smell exactly like salicylate with a twist, which my presumed methyl 2-phenoxybenzoate (2-PB) absolutely does
smell like. They smell exactly how you would imagine them based on the molecule - my presumed 2-PB is spicy like salicylate and 4-PB is somehow round
and sweet, anisic, like 4-acetphenetole (that I coincidentally also made, which makes me able to compare).
Also, I made methylparaben a while ago and it has no smell at all, so my 4-chlorobenzoic acid after reaction with phenol and base didn't turn (at least not
fully) into a 4-hydroxybenzoic acid. This is proof to me that some - maybe a lot - of my product actually is the diaryl ether carboxylic acid in these
types of reactions.
Also also the pungent smell of the chloroacids disappears during the reaction, so the chlorine is leaving the benzene ring. If it is leaving
and it is not leaving a hydroxy group behind at least some of the time, what other options besides diaryl ether are there?
Yeah, my only analytical instruments are my senses but you see that the evidence does seem to stack up.
[Edited on 17-6-2020 by michalJenco] |
The reaction is not possible as written. Draw out the mechanism for the diaryl formation- several issues arise. SnAr does exist, but I sincerely doubt
it is occuring here. I found no references that describe SnAr for that substrate under similar conditions.
|
|
|
michalJenco
Hazard to Self
 
Posts: 50
Registered: 7-2-2019
Member Is Offline
|
|
I did a melting point test in salty water (stopped at 105 °C) and my product didn't melt. Not sure how this is possible when it was liquid in the
reaction mixture at around 80.
However, seeing as my mechanism can't work, I definitely didn't make the 2,2'-oxydibenzoic acid I was going for.
However, I found this paper, which uses Cu, CuI and pyridine to condense some phenols with 2-chlorobenzoic acid. I have all of the required chemicals so I am going
to perform the synthesis (to make 2-phenoxybenzoic acid) and I will compare it with the product of my previous attempt to make this acid.
I will post the results here.
Thanks to everyone for the extensive discussion so far! Too bad it's not so easy to make diphenyl ethers.
|
|
|
michalJenco
Hazard to Self
 
Posts: 50
Registered: 7-2-2019
Member Is Offline
|
|
After some time I have an update.
I followed this paper's procedure (sci-hubbed it) with phenol and 2-chlorobenzoic acid to make 2-phenoxybenzoic acid. I did it on 3x their scale and used pyridine.HCl instead of the freebase (I used more K2CO3 accordingly). As expected, the
product is completely different from my previous attempt with the flawed procedure. I refluxed for 3.5h. My yield was only 20% while the paper is
around 50%, however. Not sure why.
I used the full 5g in the Fischer esterification with 20x molar excess of MeOH and 1.5x molar excess of H2SO4. After some 3h of reflux I got a
slightly viscous, heavier than water liquid product with a weak smell similar to methyl salicylate, but less beautiful. Also the yield was very poor,
I have less than 1g of product, not sure why again. Took a whole night to gravity filter also.
Nevertheless this is one of the most prized products I have so far, as it is such an obscure compound and I only made small amount of it.
Next time it might be interesting to decarboxylate the acid to make diphenyl ether, which as I understand it is pretty hard to make directly.
   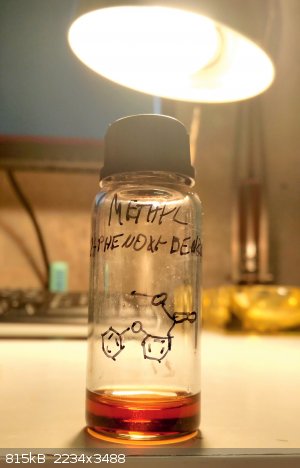
[Edited on 2-7-2020 by michalJenco]
|
|
|
| Pages:
1
2 |
|