SplendidAcylation
Hazard to Others
  
Posts: 196
Registered: 28-10-2018
Location: Starving in some deep mystery
Member Is Offline
Mood: No one I think is in my tree.
|
|
Steven's Rearrangement - Alternative strong bases to NaNH2
Hi
Having been scheming about the synthesis of N,N-dimethyl-1,2-diphenylethylamine for many months, and debating whether or not to post a thread about it
on here, I finally found a question worth asking
There have been various discussions, here and elsewhere, about this seemingly easy synthesis, but no one appears to have attempted it, and it seems to
have been overlooked that Mr Stevens himself actually synthesized this compound:
https://pubs.rsc.org/en/content/articlelanding/1932/jr/jr932...
The reaction is basically (no pun intended) the rearrangement of a dibenzyldimethylammonium halide (quaternary ammonium compound), whereby one of the
benzyl groups migrates to the alpha carbon (the CH2 on the other benzyl group), forming the desired amine in one step
Unfortunately a strong base is required for all Stevens rearrangements, but especially so for this reaction and similar ones.
The paper says that alcoholic solutions of the corresponding sodium alkoxide are ineffective, while solid sodium methoxide gives a 50% yield, and
sodium amide giving a seemingly 100% yield
I do not have any sodium, but I do have lithium and potassium, and I require the wisdom of you mad chemists as to whether I will be wasting my alkali
metals or not
Isopropanol is a weaker acid than methanol, and the basicity of the alkali alkoxides seems to go up as you go down the group, so potassium
isopropoxide sounds like it might work, being more basic than sodium methoxide
However this would require the isolation of dry potassium isopropoxide, and since there doesn't seem to be much information about this compound, I'm
not sure if it would be possible.
On the surface it seems as simple as dissolving potassium in isopropanol, followed by vacuum distillation of the excess alcohol.
My isopropanol is 99.9% pure apparently, so even if the remaining 0.01% is water, it shouldn't be a problem.
Another idea is lithium amide; it seems to be fairly doable to synthesize it, from lithium bronze as discussed here: http://www.sciencemadness.org/talk/viewthread.php?tid=10238#...
I'm not sure how basic lithium amide would be, as I can't quite figure out how its calculated; to determine the strength of a base, you can look at
the pKa of the conjugate acid, which, in the case of alkali amides, is ammonia...
But based on that alone, all the alkali amides would be equally basic, which is apparently untrue.
Phenyllithium is a much stronger base than all of the above, and it seems fairly easy to make from a halobenzene and lithium metal, which seems almost
too good to be true, but it is more nucleophilic than the other bases I think, so maybe this would cause some sort of side reaction?
The least desirable of all would be the preparation of potassium amide, as I suspect it would be quite difficult to do with gaseous ammonia due to
difficulties in drying the gas, the alternative being liquid ammonia.
Any suggestions about whether these bases would be likely to work, and whether they could actually be made, would be very much appreciated.
Thank you
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
I think I've read somewhere, that potassium tert-butoxide is a superior strong base for the stevens rearrangement, but I can't dig up again where I
saw this bit of information.
What I would like to know is, how do you plan to make the quaternary ammonium salt for this reaction?
The reaction itself is very interesting to me, and I've even considered using it myself, if it wouldn't be for the synthesis of the precursor for
which I have found no solid method with the reagents I already have on hand, and I don't want to purchase anything just for this.
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
First let me just say that Thomas Thompson is a bitching name.
Second, although I wasn't familiar with this reaction up until now, I also tend to believe that alkali alkoxides would perform pretty well(even
primary ones like EtONa).
I'm basing it primarily on the following reaction[1][2]:
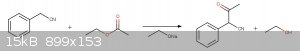
Both reactions are carried in an alcoholic solvent, traditionally with NaNH2 as a base. But more importantly, both are believed to begin with a
deprotonation of the benzylic carbon(although the functional groups attached to said carbon are quite different). Still, all in all alkoxides seems
like a fair bet to me.
---
[1]- http://www.orgsyn.org/demo.aspx?prep=CV2P0487
[2] - https://erowid.org/archive/rhodium/chemistry/p2p.phenylaceto...
[Edited on 27-8-2020 by B.D.E]
I've just read the rest of the post and saw that the article(and you) already mentioned alkoxides :S
Anyway, if you need dry alkoxides you can just add the alkali metal into some volume of heptane(/hexane/ether(probably)/etc) followed by the addition
of the alcohol. The alkoxide salt will precipitate and the hepane would help protecting it from moisture. You can then remove the excess metal with
tweezers.
Due to heptane being pretty much inhert, you might not need to vaccum filter the salt. But just keep in mind that heptane tend to form azetropes with
lots of organic compounds, so it might be a bit of pain to get rid off during the workup.
[Edited on 27-8-2020 by B.D.E]
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Nicodem mentions a good yielding rearrangement using KOtBu in refluxing toluene here: https://www.sciencemadness.org/whisper/viewthread.php?tid=13...
However I wasn't able to find that certain reference, I even wrote him just now asking for more help finding it...
But I've seen it being used at the hyperlab, for a related quat. ammonium salt, with an isolated yield of 38% of the desired tertiary amine.
This method looks definitely advantageous to me, as we know higher temperatures keep the competing sommelet-hauser product from forming.
And it allows for an in-situ formation of the quat. substrate, by simply reacting the tertiary amine with the benzyl halide in it, then adding the
base to the obtained solution, and continuing straight with the rearrangement.
I really would like to get ahold of the reference for the rearrangement... can somebody help maybe, please?
This would make me really grateful if possible.
|
|
|
SplendidAcylation
Hazard to Others
  
Posts: 196
Registered: 28-10-2018
Location: Starving in some deep mystery
Member Is Offline
Mood: No one I think is in my tree.
|
|
Thanks for the replies!
I bring news of success...
@karlos
I think potassium tert butoxide was suggested on a similar thread here, hmm.
Making the quaternary salt was surprisingly easy;
First I made benzylamine via the delepine reaction (there's a thread on it here), then did an eschweiler Clarke methylation to give
dimethylbenzylamine.
I then simply mixed the amine with a slight excess of benzyl chloride; the two compounds are miscible and mix without any obvious signs of reacting,
however, the reaction does proceed; within 30 minutes, a layer of clear gooey quaternary salt had deposited on the bottom of the beaker.
It was impossible to see until I scraped it with a spatula, so initially I thought the reaction had not started at all.
The quaternization was quite slow, but leaving it overnight was sufficient.
The impure goo was then recrystallized from acetone.
It took quite a lot of acetone; about 100ml of boiling acetone to dissolve 3g of the quat.
Upon cooling in the freezer, nothing crystallized spontaneously, but scraping the side of the beaker caused crystallization.
I got about 3g of nice white recrystallized crystals from 3g of the amine and 3g of benzyl chloride...
A 50% yield, but then again, I made no attempts to purify either the benzylamine or the dimethylbenzylamine intermediates, aside from extracting them
from the aqueous phase with DCM, drying the DCM with magnesium sulfate, then distilling off the DCM, so I wouldn't be surprised if my
dimethylbenzylamine were only 50% pure 
I have tried this whole process twice, and it is repeatable, however a few differences did arise;
The first time, the crude quaternary chloride was semi solid and able to be broken up with a spatula, it was clearly visible that the reaction had
taken place
The second time, the product was completely syrupy and it was much harder to tell that the reaction had taken place.
Also at the recrystallization stage; the first time I used fresh, dry acetone, and it took a lot to dissolve the quaternary chloride...
The second time, my acetone was slightly wet, by accident, but this time only around 20ml instead of 100ml were needed to dissolve 3g;
I was initially worried that the small amount of water might prevent the compound from crystallizing out, but it worked just fine, and with less
acetone needed...
Also, when a little cold acetone was added to the crude product, it appeared to turn from goo into fairly pure looking crystals, whereupon I heated it
and added more acetone until it all dissolved.
However, it seems likely that the goo is made of the quaternary chloride mixed with other organic stuff, and the acetone dissolved the other stuff
readily, leaving the almost insoluble product as pure crystals.
Anyway...
As for the Stevens rearrangement, I've had some success with sodium isopropoxide...
My technique is crude but seemingly effective;
One part of the quaternary chloride is placed in a test tube with one part (by mass) of the sodium isopropoxide, which is dry and alcohol free.
No solvent is used, and the reactants are mixed
I used a few hundred mgs of each
The test tube is then heated over an alcohol flame.
Nothing happens until the quaternary chloride begins to melt, whereupon a vigorous reaction takes place, with bubbling and the evolution of white
vapours and isopropanol, everything refluxes on the walls of the test tube.
If heating is discontinued immediately once the reaction has starred, it seems to continue reacting readily, however I continue heating for s few
extra seconds just to make sure the temperature is high enough to favour the Stevens rearrangement.
Once the test tube cools and the isopropanol on the sides of the tube evaporates, I'm left with some brown crystals at the bottom of the tube, with a
small amount of liquid, which I assume is a mixture of the desired amine with some dimethylbenzylamine and the sommelet Hauser rearrangement product.
Once the test tube cools, a few mls of water are added, and a definite yellow amine layer floats atop the water.
Adding saturated NaOH solution works a lot better than just water of course, but it isn't actually necessary as the mixture is already alkaline.
The immiscible layer is definitely an amine, or a mixture of them, as it dissolves when the solution is made acidic and reappears when basified
again...
Here's the original paper
https://www.thevespiary.org/rhodium/Rhodium/Vespiary/talk/fi...
[Edited on 3-9-2020 by SplendidAcylation]
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
I've got another possible alternative as a strong base: KOH in DMSO!
Stronger basicity that t-BuOK and much simpler to prepare.
See the attached document.
How do you plan to separate the sommelet-hauser and the stevens products from each other?
I've seen a paper where they attempted to do this with the differing solubility of the salts and it seemed to work quite well.
I'll load this up when I searched it out.
Attachment: trofimov1986.pdf (460kB)
This file has been downloaded 361 times
|
|
|
|