clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
a new sustainable polymer?
I was reading this review about polyacetals:
https://pubs.rsc.org/en/content/articlehtml/2019/py/c8py0121...
While a lot of it focuses on acetals incorporated into a monomer which is then esterified/etherified, there is also a novel technique where
spirocyclic polyacetals are made by rxn of pentaerythritol with dialdehydes or cyclic diketones. In particular good properties were obtained with
1,5-diethylbicyclo[3.3.0]octane-3,7-dione by Makhseed and McKeown (attached), but this is not very easy to synthesize.
However there is another similar diketone which is pretty easy to make by oxidation of isosorbide, a derivative of glucose, and what's more common than glucose?
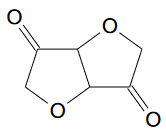
The resulting polymer looks like:
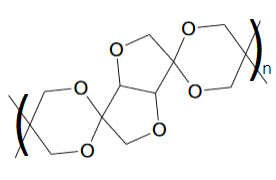
The polymerization conditions are just TsOH in toluene at reflux. The twisted structure of the polymer should promote solubility according to the
findings with other spirocyclic polyacetals. However, I'm not sure if the steric hindrance will allow you to make this. Any thoughts?
Pentaerythritol and isosorbide are not particularly expensive. I'm guessing chromate would be fine for oxidizing isosorbide but it is important to
avoid further dehydrogenation to furo[3,2-b]furan-3,6-diol.
Attachment: makhseed1999.pdf (111kB)
This file has been downloaded 427 times
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Bromolone
Harmless

Posts: 20
Registered: 16-9-2019
Member Is Offline
Mood: Aldehydic
|
|
The diketone polymer is well-susceptible to ring-opening in strongly acidic & alkaline conditions(although this requires some catalysts) as far as
I can structurally interpret.
Synthesis is an excellent idea, but the monomer described in the paper you referenced seems fairly inert to excessive oxidations, while
dehydrogenation in chromate for isosorbide may rip stuff apart.
There's a sound lack of research on the synthesis of structurally exotic compounds, anyways. 
[Edited on 4-7-2020 by Bromolone]
Good documentation is key to good chemistry and software alike.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Cellulose.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
It doesn't appear that there are any reports using dichromate on isosorbide and Bromolone probably has a point about overoxidation.
However, TEMPO seems to work okay:
https://pubs.rsc.org/en/content/articlehtml/2014/gc/c3gc4185...
As far as I can tell laccase is just used in the article to regenerate TEMPO+. Apparently, isosorbide diketone is already used as an intermediate to
generate the corresponding diisocyante via reductive amination and COCl2. HClO/TCCA/NBS etc won't work because they chlorinate the ketone.
Ceccheto et al reported in 2001 a bimetallic system of manganese and cobalt nitrates can aerobically regenerate TEMPO+ using oxygen from air at
standard conditions. This could work -- attached.
Attachment: cecchetto2001.pdf (49kB)
This file has been downloaded 245 times
Unfortunately cellulose acetate is pretty weak and the other cellulose derivatives are all unstable (formate, carbamate) or even weaker (methyl,
ethyl, propionate, butyrate). Ionic liquid lyocell is pretty cool though.
[Edited on 8-7-2020 by clearly_not_atara]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|