Draeger
Hazard to Others
  
Posts: 185
Registered: 31-1-2020
Location: North-Rhine Westfalia, Germany
Member Is Offline
Mood: Slowly getting ready for new projects
|
|
Synthesis of dicyclopentadiene?
Is it possible to synthesize dicyclopentadiene? I've only heard of it being made from crude oil, but never really synthesized. Is it just too hard to
do? Dicyclopentadiene is pretty hard to obtain, I have only found one supplier which sells 100ml for 80€ with 20€ shipping, and I thought maybe
it'd be cheaper to synthesize it, or at least an interesting procedure.
I'm far from ready to work with it, but I was curious since at some point I do plan to work with it.
Collected elements:
Al, Cu, Ga, C (coal), S, Zn, Na
Collected compounds:
Inorganic:
NaOH; NaHCO3; MnCl2; MnCO3; CuSO4; FeSO4; aq. 30-33% HCl; aq. NaClO; aq. 9,5% ammonia; aq. 94-96% H2SO4; aq. 3% H2O2
Organic:
citric acid, sodium acetate, sodium citrate, petroleum, mineral oil
|
|
|
Tdep
National Hazard
   
Posts: 516
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
I've thought about this too, and the sad part is the most cost effective + least transport regulation violations way I've found is... destructive
distillation of ferrocene?
You can buy ferrocene reasonably non-expensively at the 500g level, and its shipped as a dry powder, and there's probably maybe even a nice way to
break it up chemically, although just heating it to oblivion will probably work fine (maybe?)
|
|
|
Tdep
National Hazard
   
Posts: 516
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
Ok so the temperature is quite hot, you'll have a lot of subliming/boiling ferrocene so it'll be messy, and your yields might be trash because a lot
of your product turns into methane, but sure, it'll work I guess
Reference https://onlinelibrary.wiley.com/doi/abs/10.1002/cvde.2005064...
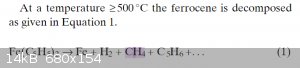
|
|
|
Draeger
Hazard to Others
  
Posts: 185
Registered: 31-1-2020
Location: North-Rhine Westfalia, Germany
Member Is Offline
Mood: Slowly getting ready for new projects
|
|
Oh. Are there any other methods?
I was also wondering if there was a way to synthesize it from a precursor that doesn't have dicyclopentadiene as a precursor as well? I guess it
really wouldn't be as effective as other methods, but it might still be interesting?
Collected elements:
Al, Cu, Ga, C (coal), S, Zn, Na
Collected compounds:
Inorganic:
NaOH; NaHCO3; MnCl2; MnCO3; CuSO4; FeSO4; aq. 30-33% HCl; aq. NaClO; aq. 9,5% ammonia; aq. 94-96% H2SO4; aq. 3% H2O2
Organic:
citric acid, sodium acetate, sodium citrate, petroleum, mineral oil
|
|
|
Prepic
Harmless

Posts: 31
Registered: 23-4-2019
Member Is Offline
|
|
The best way I can think of is starting from adipic acid to form cyclopentanone. From there:
Reduction to cyclopentanol; C5H10O
Dehydration to cyclopentiene; C5H8
Followed by Br2/ H2O to form C5H9OBr
And then lastly dehydration and dehalogenation into cyclopentadiene.
Lengthy, but it should all be relatively OTC at least.
|
|
|