Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
Det caps in air bags
So,
To preface i know nothing about energentics, so i apologise in advance if anything i ask that seems obvious.
I cannot find much on the composition of the det caps in air bags that generate the heat needed to set off the sodium azide. Anyone know what this
maybe.
The caps are electrically intiated, does this mean they are not shock sensitive?
Finally can someone point to a description of dismantling an air bag that they can vouch for, as in having done something similar or the same
themselves.
Finally the entire device (the one i am holding is from a steering wheel) is made of heavy (8mm or so) aluminium, is a circular thing about an inch
thick with a series of holes around t h e edge. These holes are covered internally with a heavy al foil. I assume these foiled holes act as the points
for the exhaling nitrogen burst. And that the two sides of the thick body remain unaffected after set off. Meaning it could simply be pierced to drain
out the azide, asssuming its a free flowing powder. Is there a problem with this logic. I would pierce with a heavy syringe needle
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
There are several types of airbags, some of them do not have NaN3 in them, because it is toxic. I suggest reading Wiki article , and checking Your particular airbag. I believe there is no detonator as such in those, but an e-match, but I have no experience
with airbags nor I have extended knowledge on subject.
I attached a picture of airbags structure.
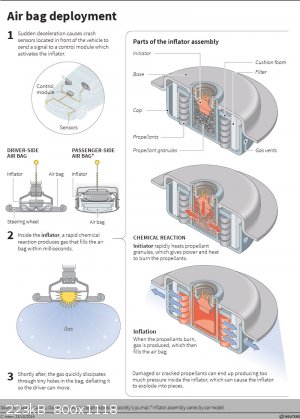
|
|
|
Deathunter88
National Hazard
   
Posts: 507
Registered: 20-2-2015
Location: Beijing, China
Member Is Offline
Mood: No Mood
|
|
To clarify, most modern airbags no longer contain sodium azide. Also the propellant will be in the form of pellets instead of a free flowing powder.
If you just want the sodium azide it is cheaper and safer to just buy some on amazon with free prime shipping: https://www.amazon.com/HiMedia-GRM123-100G-Sodium-Azide-100/...
17USD for 100g
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
Thanks guys,i should have carified, the device is fro the ate90's, states 'containds sodium azide and potasi u m nitrate on it and i don't ne ed or
want then azide, just bored in hard lockdown.
An ematch, thats a new one, wonder if they will release an ee eventually?
|
|
|
aromaticfanatic
Hazard to Others
  
Posts: 173
Registered: 10-9-2019
Member Is Offline
|
|
Quote: Originally posted by Panache  | Thanks guys,i should have carified, the device is fro the ate90's, states 'containds sodium azide and potasi u m nitrate on it and i don't ne ed or
want then azide, just bored in hard lockdown.
An ematch, thats a new one, wonder if they will release an ee eventually? |
NaN3 has a solubility of 38.9g/100ml of water.
KNO3 has a solubility of 13.3g/100ml of water.
There is a potential for recrystallization purification but it'd be tedious and inefficient. I suggest looking for a solvent that will easily dissolve
the azide but not the nitrate.
Best of luck
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
I cut open an airbag several years ago and I found what's left of it after I retrieved the sodium azide from it in the back of a cupboard in a sealed
bag today.
I remember carefully using a hacksaw to cut the four notches so that the top could be unscrewed. You can see where the cuts were made in the photo.
The top (which I can't find) was unscrewed slowly using a strong vice grip wrench. Once unscrewed you can pull the initiator out and it is safe.
The pellets are black in colour and contained in a thin tin circle which can be easily pierced to retrieve them. This can be seen in the photo too.
I remember dissolving them in water to make lead azide and the black material remaining undissolved and being some sort of metal as it all attached to
the stir bar and was hard to remove.
The initiators are quite powerful, i believe allchemystery has a video setting one off.
Hope that helps slightly, it was a long time ago I did it
[Edited on 11-10-2020 by greenlight]
 
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
OldNubbins
Hazard to Others
  
Posts: 136
Registered: 2-2-2017
Location: CA
Member Is Offline
Mood: Comfortably Numb
|
|
Useless information:
I used to live near the TRW airbag factory in Arizona. We called it the 'Pipe Bomb Factory". There was at least one incident every year someone would
get injured or killed, they never did seem to be able to handle the azide safely. Every summer when the monsoons came, the entire plant would have to
shut down if there was a lightning strike within 1 mile. There was an Air Force base nearby as well, and depending on conditions, operations would
halt if any air traffic was overhead. That place was a hot mess...
|
|
|
chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Holy crap! That reminds me of the teflon tape factory my brother used to work in. There were fires all the time, a few of his co-workers died of
cancer, there was a leak from the ceiling and an oily liquid rained down on everyone in the room, a huge tank full of chemicals fell over right next
to my brother and almost crushed 2 employees, if you touched the wrong part of some of the machines they would shock you violently and really
painfully, and the jacket he wore to work was always covered in little speckles of what i guess was teflon.
We always had teflon tape though (:
|
|
|
Yankeehater2679
Harmless

Posts: 9
Registered: 14-5-2018
Member Is Offline
|
|
Ik this a little off topic but is it possible to use the NaN3+kno3 mix as a detanator, or is not strong enough or would u just need to much?
|
|
|
chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Quote: Originally posted by Yankeehater2679  | | Ik this a little off topic but is it possible to use the NaN3+kno3 mix as a detanator, or is not strong enough or would u just need to much?
|
Sodium azide is a very powerful detonator on its own. What is the reasoning for adding KNO3?
|
|
|