Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
The reaction of guanidine with alkyl thiuronium salts
I have been trying to prepare biguanide for some time. There are numerous published methods but in essence they boil down to two systems: 1- fusion of
ammonium salts with dicyandiamide and 2- the guanylation of guanidine.
I have not had much luck with the dicyandiamide fusion routes. I have tried ammonium chloride, ammonium iodide, ammonium nitrate and ammonium acetate
mixtures. The best yield I have achieved was with ammonium nitrate at about 8% but fusing this salt with dicyandiamide is somewhat scary (think
Beruit). I tried using a eutectic mix of ammonium nitrate with ammonium acetate but this reduced the yield greatly. The ammonium chloride route is
tough because the melting point of the mixture is well above the optimum for biguanide (about 130 C) and with ammonium iodide the work up is messy and
complex. The main product of all these reactions are guanidine salts.
So I decided the high yielding reaction of O-methyl-isourea with guanidine looked much better. The problem is that isoureas are hard to prepare ie
dimethyl sulphate on urea or sodium methoxide/methanol on cyanamide.
However, alkyl thio-isoureas are easily prepared from alkyl halides and thiourea. So I decided to try the reaction of ethane 1,2-bis(thiuronium
bromide) on various forms of guanidine. I used this bis-thioisourea because the byproduct is a mercaptan and the ethylene-thioglycol is much less
volatile and smelly than methyl thiol.
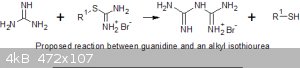
There is a fairly rapid reaction but no thioglycol is produced (no adverse smell at all!) and tests with ammoniacal copper sulphate do not give a
violet biguanide complex ppt. Sometime its pale blue copper hydroxide and other time black (copper sulphide?). There is produced however, a large
amount of pure white ppt.
If the ethane bis(thiuronium) salt is reacted with excess ammonia guanidine results, with aniline the product is phenyl guanidine and hydrazine
hydrate yield aminoguanidine. So the reaction of guanidine to produce biguanide is not without precidence.
Does anyone have any idea about the reactions of ethanedithiol? Could it react with the biguanide or am I getting a completely different reaction. The
white product once formed is resistant to both dilute NaOH and HCl even on brief boiling and is insoluble in both suggesting that it is a neutral
compound. After filtering of the weakly alkaline solution gives a black (sulphide?) ppt with amminocupric sulphate but once acidified with HCl give a
very pale gelatinous ppt reminescient of copper silicate.
Dithioglycol reacts with ketones to give cyclic 2,2-dialkyl-1,3-dithioles; could I be getting something similar?
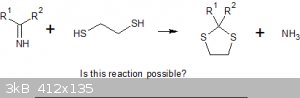
[Edited on 7-10-2020 by Boffis]
[Edited on 7-10-2020 by Boffis]
|
|
|
Dr.Bob
International Hazard
    
Posts: 2658
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
I am not an expert on guanidines at all, but I did run an experiement similair to the scheme one above with a subst. guanidine and the S-Me thiourea
(I think I mae it from thiourea and MeI, if my poor memory is still working.) I don;t remember the methyl thiol smell being a problem (but I was ina
hood), and I think the reaction did work pretty well. The real problem is that the products are all very water soluable, so hard to do any
extractionos, chromatopgraphy, etc, you have to ppt them or recrystallize them to purify. If you want, I can try to find more details, but it
might take a little while.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Thanks Dr.Bob; I might try this again with methyl isothiuronium bromide as I have a bottle of methyl bromide in the freezer. In theory (wondeful thick
theory!) I should be able to distill the methyl thiol from the reaction mixture just leaving the biguanide bromide which I can then ppt with copper
sulphate. The recover of the biguanide from there is not too difficult.
I have examined the aqueous phase and the white solid and I am no wiser! The white solid is a mixture of two compounds one soluble in boiling alcohol
and one insoluble. The latter has a Mp about that of boiling alcohol, it is not decomposed even on boiling with 40% sodium hydroxide! The aqueous
phase was evaporated down and deposits a lot of salt crystals but the residue has retained the ability to precipitate Cu, almost white gel with
neutral CuSO4 but greenish black with cuprammino sulphate solution. I am presuming that the sulphur has ended up in the aqueous phase but in what form
is not clear. No biguanide formed though.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2658
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
I think all I did was evaoprate the reaction and the methyl thiol was gone. My reactions were only on a 100-1000 mg scale, so the amount of thiol
was not enormous. tBu thiol is way worse, trust me. Plus everyone associates that with gas leaks, so even traces of that can cause a panic, but
methyl is so volatile that small amounts seem to vanish. Methyl disuilfide, however is twice the mw so much lower bp, but gives off contant traces
of the thiol, so it is a pain to use. Good luck. Methyl bromide should work fine with sulfur, it is pretty reactive.
|
|
|