| Pages:
1
2 |
Fluorite
Hazard to Others
  
Posts: 132
Registered: 26-12-2018
Location: United Arab Emirates
Member Is Offline
|
|
ammonium nitrate to nitric acid and ammonia? with phosphoric acid catalyst!
Is there a cheap way to make nitric acid and ammonia from ammonium nitrate?
Electrolysis can be tricky and complicated (btw can I use gold to electrolyse sulfuric acid with no chlorides?)
Thermal decomposition will produce nitrous oxide which is useless and doesn't react with anything except sodium amide and fire
I was wondering if adding a catalyst like phosphoric acid and distill HNO3 at 120c this should work
Ammonium phosphate decomposes to ammonia and phosphoric acid at 200c! It's totally catalytic
Any ideas?
[Edited on 8-11-2020 by Fluorite]
[Edited on 8-11-2020 by Fluorite]
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
They thermally decomposed few kilotons of AN in Beirut and even in best case scenario it would produce only nitrous oxide like you said so I don't
suggest that way.
Cheap? Calcium hydroxide + ammonium nitrate = calcium nitrate + ammonia, although the actual kinetics might be not so shake-and-bake due to the
insolubility of Ca(OH)2. Calcium nitrate goes thermal decomposition into NO2, which can be led to water to generate HNO3, but this process is not 100%
but more like 50% efficient.
Sodium bisulfate would generate ammonium sulfate and HNO3.
HCl is supposed to react with nitrates to generate HNO3 and a chloride.
Sulfuric acid process is the gold standard, and if you got access to commercial quantities, it's a good choice. You need to purchase 25-200L though.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
If you have the kit and the other raw materials.
Treat a solution of Ammonium nitrate with sodium hydroxide to produce ammonia and sodium nitrate (and water).
Heat it to drive off the ammonia and dissolve that in water for later use.
Dry out the sodium nitrate and add it to sulphuric acid and then distill out the nitric acid.
In principle, you can do this the other way round- add the ammonium nitrate to sulphuric acid and distill out the nitric acid, then add sodium
hydroxide to the ammonium sulphate produced.
My prefered option doesn't involve heating ammonium nitrate.
|
|
|
Fluorite
Hazard to Others
  
Posts: 132
Registered: 26-12-2018
Location: United Arab Emirates
Member Is Offline
|
|
YAAAAAAS I found it
If the salt is formed by a non-volatile acid, then heating proceeds with the decomposition of the ammonium salt: N H 4 H 2 P O 4 → N H 3 + H 3 P O 4
Source:
https://ru.m.wikipedia.org/wiki/Аммоний
OMGGGGGGGG HOLY COW that's it I'm the smartest 
I'll try this asap and update you guys
I'll add phosphoric acid to ammonium nitrate and distill HNO3 okay this should work!
And then heat ammonium phosphate until decomposition to make ammonia Gas!! Phosphoric acid is just the catalyst!
You should give me an award or something
[Edited on 8-11-2020 by Fluorite]
|
|
|
Fluorite
Hazard to Others
  
Posts: 132
Registered: 26-12-2018
Location: United Arab Emirates
Member Is Offline
|
|
I'M A F-ING GENIUS
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
If it works that way it is nice, because phosphoric acid is cheap and if it can be regenerated simply by heating off the ammonia, it saves a lot of
reagents.
The most arduous way by far presented here is to treat ammonium nitrate with sodium hydroxide and then with sulfuric acid. Both reagents are
relatively expensive and for some their acquisition might be limited. For example a 100mol batch batch of ammonium nitrate one would need 4kg of NAOH
and then 10kg of sulfuric acid, and at technical grade prices this would yield about 100-150€ + several hours of work.
Yeah, I'm a fanatic of process economics.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Hot phosphoric acid destroys glassware.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Just like ammonium nitrate.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Yep, but it is always good to pick all the optimal choices instead of just throwing hands up in the air and ordering everything in ACS grade in 200mL
bottles.
AN can usually be sourced somewhat reasonably from many products after 2- or more step purification process, or at least it can be used as a source of
nitrate either with acid or by recrystallizing as KNO3.
|
|
|
Fluorite
Hazard to Others
  
Posts: 132
Registered: 26-12-2018
Location: United Arab Emirates
Member Is Offline
|
|
Yea sure ammonium nitrate isn't available commercially but a friend of mine is a farmer and sometimes he can bring me a 2kg
Also I should try this and maybe if I make a copper flask cuz copper phosphate is insoluble this should be useful for protecting the flask as
unionised stated phosphoric acid can dissolve glass but as far as I know very slowly
|
|
|
Fluorite
Hazard to Others
  
Posts: 132
Registered: 26-12-2018
Location: United Arab Emirates
Member Is Offline
|
|
BUT IF THIS WORKS! Oh god why I feel so excited am I the first person who discovered this!!!
LOOK!!!!
This can make many things
Ammonium bicarbonate and sodium chloride = ammonium chloride + sodium bicarbonate OKAY? And then ammonium chloride + phosphoric acid = HCL! And
ammonium phosphate which as I said earlier can be decomposed to phosphoric acid and ammonia! So using just Carbon dioxide and sodium chloride
YOU CAN MAKE HCL AND SODIUM BICARBONATE THIS IS TOTALLY CATALYTIC!!!!!!!
I'M A F-ING GENIUS SORRY NOT SORRY But I need help *^* +216 98462822 my WhatsApp
[Edited on 8-11-2020 by Fluorite]
|
|
|
ArbuzToWoda
Hazard to Self
 
Posts: 98
Registered: 15-7-2020
Member Is Offline
|
|
Quote: Originally posted by Fluorite  | BUT IF THIS WORKS! Oh god why I feel so excited am I the first person who discovered this!!!
LOOK!!!!
This can make many things
Ammonium bicarbonate and sodium chloride = ammonium chloride + sodium bicarbonate OKAY? And then ammonium chloride + phosphoric acid = HCL! And
ammonium phosphate which as I said earlier can be decomposed to phosphoric acid and ammonia! So using just Carbon dioxide and sodium chloride
YOU CAN MAKE HCL AND SODIUM BICARBONATE THIS IS TOTALLY CATALYTIC!!!!!!!
I'M A F-ING GENIUS SORRY NOT SORRY But I need help *^* +216 98462822 my WhatsApp
[Edited on 8-11-2020 by Fluorite] |
So far you're posing to be quite a clown. Such ionic reactions are equlibriums and as both ammonium chloride and sodium bicarbonate are well soluble
in water that's not the way to go.
|
|
|
Fluorite
Hazard to Others
  
Posts: 132
Registered: 26-12-2018
Location: United Arab Emirates
Member Is Offline
|
|
Quote: Originally posted by ArbuzToWoda  |
So far you're posing to be quite a clown. Such ionic reactions are equlibriums and as both ammonium chloride and sodium bicarbonate are well soluble
in water that's not the way to go. |
Don't hate me for discovering a new processes ¯\_(๑˙❥˙๑)_/¯. You should be supportive
Also sorry but you're wrong
https://en.m.wikipedia.org/wiki/Solvay_process
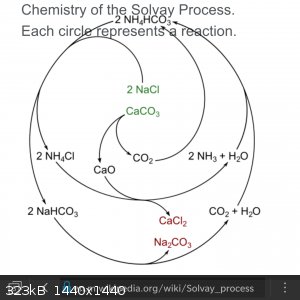
[Edited on 8-11-2020 by Fluorite]
[Edited on 8-11-2020 by Fluorite]
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
To be honest, these reactions are basic petty chemistry and probably invented back in the 1800's when everything else was discovered too.
It doesn't mean they're not useful, they are very indeed.
If both ions are soluble and their solubilities don't greatly differ, it is hard to separate them. It is easy to crash out calcium carbonate from
equation, or if you mix ammonium nitrate and potassium chloride, you would be able to extract potassium nitrate crystals when cooling the solution,
while both potassium chloride, ammonium nitrate and ammonium chloride ion pairs will remain in solution. This is actually my preferred way to generate
KNO3 as a useful nitrate source as a reagent use from guess-game nitrate mixtures which are adulterated by evil chemists.
[Edited on 8-11-2020 by Fyndium]
|
|
|
ArbuzToWoda
Hazard to Self
 
Posts: 98
Registered: 15-7-2020
Member Is Offline
|
|
Quote: Originally posted by Fluorite  |
[/rquote]
So far you're posing to be quite a clown. Such ionic reactions are equlibriums and as both ammonium chloride and sodium bicarbonate are well soluble
in water that's not the way to go.[/rquote]
Don't hate me for discovering a new great and amazing processes ¯\_(๑˙❥˙๑)_/¯. You should be supportive
Also sorry but you're wrong
https://en.m.wikipedia.org/wiki/Solvay_process
[Edited on 8-11-2020 by Fluorite] |
I'm not hating you. Just this self-praise without actual experimentation is unjustified.
The Solvay process is something entirely different. First of all, it's an industrial method that works on huge scales and therefore may accept
simplifications. Note that due to hydrolysis the solution of NH4Cl will be weakly acidic, thus possibly breaking the basic buffer that is
required for the bicarbonate precipitate out. Also the subsequent reaction would require you to either melt the two solids together or use commercial
85% phosphoric acid and that would lower the yield significantly due to fantastic solubility of HCl in water. I'm not saying it's impossible, but it
is a shit ton of hassle to get such a basic reagent.
|
|
|
Fluorite
Hazard to Others
  
Posts: 132
Registered: 26-12-2018
Location: United Arab Emirates
Member Is Offline
|
|
THANKS guys! please don't hesitate to try this yourself if you have a broken flask or something because again phosphoric acid can dissolve glass
Also I'm kidding when I said I'm the smartest person on earth it's a freaking joke so nobody can sue me. Everything I said was well thought out and
genius but that doesn't mean it's true. None of it is confirmed (but that doesn't take away from the fact that Fluorite is a genius and deserves a lot
of awards).
Okay bye lol stay woke XD
|
|
|
ArbuzToWoda
Hazard to Self
 
Posts: 98
Registered: 15-7-2020
Member Is Offline
|
|
Quote: Originally posted by Fluorite  | THANKS guys! please don't hesitate to try this yourself if you have a broken flask or something because again phosphoric acid can dissolve glass
Also I'm kidding when I said I'm the smartest person on earth it's a freaking joke so nobody can sue me. Everything I said was well thought out and
genius but that doesn't mean it's true. None of it is confirmed (but that doesn't take away from the fact that Fluorite is a genius and deserves a lot
of awards).
Okay bye lol stay woke XD |
Well okay, that's just irritating. Good of you to be aware of that, though.
|
|
|
Fluorite
Hazard to Others
  
Posts: 132
Registered: 26-12-2018
Location: United Arab Emirates
Member Is Offline
|
|
Wait when hot Phosphoric acid react with glass what happens? Like silicone phosphate or what?
|
|
|
ArbuzToWoda
Hazard to Self
 
Posts: 98
Registered: 15-7-2020
Member Is Offline
|
|
https://www.sciencedirect.com/science/article/abs/pii/002230...
2 seconds in google.
|
|
|
Fluorite
Hazard to Others
  
Posts: 132
Registered: 26-12-2018
Location: United Arab Emirates
Member Is Offline
|
|
I saw this but can silicon phosphate make a protective layer and stop further reactions
|
|
|
ArbuzToWoda
Hazard to Self
 
Posts: 98
Registered: 15-7-2020
Member Is Offline
|
|
Yes, but at the same time it's formation destroys the structure of glass. So that could lead to the same thing as sodium hydroxide in NurdRage's
process.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I'm sure this has been tried in the 1800's. The fact there isn't any Google result popping up immediately makes me wonder how well this process runs.
Please try and report back to us.
[Edited on 8-11-2020 by Tsjerk]
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
That explains why that's not used to make nitric acid.
|
|
|
Fluorite
Hazard to Others
  
Posts: 132
Registered: 26-12-2018
Location: United Arab Emirates
Member Is Offline
|
|
Oh well I guess I should start collecting beer bottles from the beach and drill a hole and make a crude distillation setup  if phosphoric acid dissolve glass faster than I expect it's fine because many jerks
keep throwing bottles and I get them for free. The trick is to heat the bottom without cracking :/ I guess I'll try oil bath if phosphoric acid dissolve glass faster than I expect it's fine because many jerks
keep throwing bottles and I get them for free. The trick is to heat the bottom without cracking :/ I guess I'll try oil bath
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Fluorite  | Yea sure ammonium nitrate isn't available commercially but a friend of mine is a farmer and sometimes he can bring me a 2kg
Also I should try this and maybe if I make a copper flask cuz copper phosphate is insoluble this should be useful for protecting the flask as
unionised stated phosphoric acid can dissolve glass but as far as I know very slowly |
Is copper phosphate soluble in hot phosphoric acid?
I suggest you ask your farmer friend about sulphuric acid and even ammonia.
But for those of us who have to rely on buying stuff...
|
|
|
| Pages:
1
2 |