symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
The Mad Science Report 2020
Quote by: Chemetix
Physicists take their superiority from working with first principles and mathematical logic.
Chemistry gets its results from the messy chaotic world of reality.
Starting a lab
Basic requirements
First, one will need to choose a place generally away from dwelling space. A shed is a good choice, as it offers natural ventilation which prevents
the build-up of flammable vapors and toxic compounds. However, unless you live in a geographical area where winters are mild or non-existent, sheds or
other locations with natural ventilation may not be a good choice for your lab, as it will get too cold inside and this will interfere with various
reactions or other processes.
Furniture, like cabinets, chairs, closets, cupboards, shelves and tables are needed in the lab to store reagents and lab apparatus, as well as a
working bench for the reactions.
Reagents are necessary when doing any form of chemistry. Important reagents include:
Acids: acetic acid, formic acid, hydrochloric acid, nitric acid, sulfuric acid
Bases: sodium hydroxide, potassium hydroxide, calcium hydroxide, ammonia
Salts: sodium chloride, calcium chloride, copper sulfate, potassium nitrate, ammonium nitrate, sodium bicarbonate, magnesium sulfate
Metals: aluminium, copper, iron, magnesium, sodium, zinc
Nonmetals: bromine, chlorine, iodine, phosphorus, sulfur
Organic solvents: acetone, chloroform, diethyl ether, ethanol, ethyl acetate, isopropanol, methanol, mineral oil, toluene, xylene
Inorganic solvents: water (tap or distilled)
pH indicators: phenolphthalein, methyl orange, litmus
Oxidizers: hydrogen peroxide, sodium hypochlorite
Electricity and water are a must. While you can use generators if the location is not near the grid, they tend to be noisy and require fuel. You can
also rely on alternative power sources, like solar or wind, if possible.
If distilled water is required, you can hook up a water distiller to your tap water. If you cannot get water from tap, you can use barrels or tanks
full of water.
Fuel source You can get it by either connecting to the gas grid or using propane tanks.
Your lab should be connected to a drain or, if that is not possible, to a waste container.
Disposal tanks
Corrosive or hazardous products that cannot be properly neutralized quickly, contain valuable or toxic metal ions or require special disposal are to
be stored in chemical resistant bottles, usually glass, generally in a ventilated area. These waste tanks must be properly labeled to prevent any
accidental mixing of incompatible chemicals.
NEW PROCEDURES
Make Sodium Metal with Menthol
It is a great project and achievement for the experienced amateur chemist. An efficient way to obtain sodium metal is to heat sodium hydroxide with
magnesium in mineral oil above 200°C, in the presence of a tertiary or sterically bulk secondary alcohols, like t-butanol and menthol. The yield of
this route if done right can be as high as 90-95%, though the process takes hours to completion.
Make Nitric Acid by Thermal Decomposition of Copper Nitrate
Copper sulfate is reacted with calcium nitrate the resulting calcium sulfate is filtered from the solution as copper nitrate remains. This reaction
is a cheap source of nitrogen dioxide, which can be bubbled through water to generate nitric acid. The gas can also be bubbled into hydrogen peroxide
producing a higher yield.
Nitric acid is typically made by the method of potassium nitrate and sulfuric acid but this method can be improved as a more recyclable reactant by
using copper nitrate and sulfuric acid this generates Nitric acid and copper sulfate byproduct. The copper sulfate can be processed to recover
sulfuric acid and copper metal by electrolysis.
For dilute nitric acid
Magnesium nitrate or sulfuric acid can be used as a drying agent to produce red or white fuming nitric acid.
Technochemistry
Nitric acid from ammonia Oswalt style (without platnium)
This process uses urea as a source of ammonia which is heated till it decomposes into ammonia and carbon dioxide, oxygen is added to the system using
an Air compressor. A condenser is used to dry the ammonia/air stream. The ammonia air mixture is fed into a Quartz tube with a 8mm ID that is heated
with nichrome wire heating element. The reaction chamber contains NiO or CoO as a Catalysis that are supported bed of Sand or a red brick chunks. The
ammonia is oxidized to nitrogen monoxide. The Quartz tube runs into an Absorption tower (converted 2L sep. funnel water with broken glass to increase
surface area. which admits air via the custom condenser fitting. The absorption tower oxidizes the nitrogen monoxide to nitrogen dioxide which
combines with the moisture of the reaction to produce nitric acid. This condensate runs into a reservoir to collect the Nitric acid the excess gasses
run to a vertical anti suck back column before running along a small tube to the base of the tower.
Sulfur trioxide
The preparation of Sulfur Trioxide is extremely dangerous and should only be attempted by chemists with experience working with hazardous volatile
reagents.
Pyrolysis of Sodium bisulfate first evolves water, forming sodium pyrosulfate, which then decomposes above 460 °C to sodium sulfate, releasing Sulfur
Trioxide this reaction temperature can be lowered to 150°C if concentrated sulfuric acid is added to the Sodium Bisulfate.
Pyrolysis of transition metal sulfates can also be used to make sulfur trioxide. Heating iron(III) sulfate at 480 °C it decomposes produces mostly
sulfur trioxide. Heating copper sulfate above 560 °C yields sulfur trioxide. Heating iron(II) sulfate at 700 °C with carbon yields iron(III) oxide,
sulfur dioxide and sulfur trioxide. Aluminium sulfate also works, though the decomposition temperature is slightly higher.
Sulfur trioxide can also be obtained by oxidizing sulfur dioxide in the presence of several metal oxides, such as copper(II) oxide at high
temperatures or chromium(III) oxide (at temperatures between 180-400 °C).
Adding phosphorus pentoxide to extremely concentrated sulfuric acid will release sulfur trioxide, which can be extracted via distillation.
Metaphosphoric acid can also be used instead of the pentoxide.
Sulfuric acid
A solution of copper sulfate can be electrolyzed with a copper cathode and platinum/graphite anode to give spongy copper at cathode and evolution of
oxygen gas at anode, the solution of diluted sulfuric acid indicates a completed reaction when it turns from blue to clear (production of hydrogen at
cathode is another sign):
When applying power, the current should be adjusted so that corrosion at the positive terminal and bubbling at the negative terminal are both
minimized. The bubbling at the negative terminal is hydrogen production and that's wasted energy that should have gone into reducing the copper
sulfate..
Keep in mind that it will take a long time for the solution to go clear. if after filtering it's still blue, you'll need to keep electrolyzing it.
You'll need over 60 amp hours of current per mole of copper sulfate, and usually more since the process is not very efficient
Make Sulfuric acid (metabisulfite/oxidizer method)
Sodium metabisulfite is placed at the bottom of a beaker, and 12.6 molar concentration hydrochloric acid is added. Sodium metabisulfite upon reaction
with acid will generate sulfur dioxide.The resulting gas is bubbled through nitric acid, which will release brown/red vapors. The completion of the
reaction is indicated by the ceasing of the fumes. The sulfur dioxide can also be dissolved in a hydrogen peroxide solution to form sulfuric acid This
method of oxidizing sulfur dioxide in solution does not produce an inseparable mist, which is quite convenient.
Make Sulfuric Acid by the Copper Chloride Process
Take a solution of copper (II) chloride and bubble sulfur dioxide into it until most of the copper (II) chloride is converted into copper (I)
chloride. This reaction also converts the sulfur dioxide into sulfuric acid and produces hydrochloric acid. Now the copper chloride can be regenerated
by bubbling air into the mixture until the copper chloride dissolves again. This cycle can be repeated. When you want to isolate the sulfuric acid you
forgo air infusion for that step and filter off the precipitated copper chloride. Then you distill off the hydrochloric acid and water from the
filtrate. The sulfuric acid left behind will precipitate out most of the remaining copper salts and when filtered will give you relatively pure
sulfuric acid with some water and minor copper contamination. The copper chloride can be recombined with all the hydrochloric acid from before and the
cycle repeated.
Sulfuric acid (electrobromine process)
make sulfuric acid from sulfur and water using electrolytically generated bromine as the catalyst.
The electrobromine method, which employs a mixture of sulfur, water, and hydrobromic acid as the electrolytic solution. The sulfur pushed to bottom of
container under the hydrobromic acid solution, then copper cathode and platinum/graphite anode are used with cathode near surface and anode at bottom
of electrolyte to apply the current. This may take longer and emits toxic bromine/sulfur bromide vapors, but reactant acid is recyclable, overall only
sulfur and water converted to sulfuric acid (omitting losses of acid as vapors)
Start with 16g of sulfur in a 250 ml beaker. Install 5 lantern battery carbon electrodes bound together to increase surface area for the anode and a
copper wire for the cathode. To this setup we add 200mL of 5M hydrobromic acid. The copper cathode is readjusted to be as physically close to the top
of the electrolyte as possible while still being immersed in it. A current is then applied of less than 2 amps. The electrolysis is performed for 40
hours with occasional stirring and addition of water to make up for evaporative loses.
At the end of the run, the solution is filtered and then refluxed for 30 minutes to drive the conversion to completion. The solution is then distilled
to first recover water, then hydrobromic acid and finally sulfuric acid.
Hydrobromic acid
The reaction between Sodium bromide and diluted sulfuric acid produces hydrobromic acid the solution is cooled and the sodium sulfate precipitate is
filtered. Do not to use concentrated sulfuric acid or another oxidizing acid that will oxidize bromide to elemental bromine.
Hydrochloric acid
One way of synthesizing HCl is to react a mixture of sodium chloride and sodium bisulfate, or sulfuric acid. The mixture is heated and the resultant
hydrogen chloride gas is then bubbled through a solution of cold distilled water to produce hydrochloric acid.
Small amounts of hydrogen chloride for laboratory use can be generated in an HCl generator by dehydrating hydrochloric acid with either sulfuric acid
or anhydrous calcium chloride.
purification crude hydrochloric acid by using the two-container technique.
The process takes a week or two to finish, and the resulting reagent-grade acid is about 5 molar rather than the 12 molar typical of commercial
concentrated hydrochloric acid. Still, it's extremely pure and concentrated enough for most purposes.
LAB TECHNIQUES
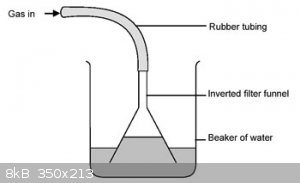
A funnel-and-beaker trap is a device for dissolving highly soluble gases in water. The trick with these gases, such as ammonia and hydrogen chloride,
is that they cannot be dissolved in water by simply bubbling them through it: the high solubility results in the water rushing back into the reaction
vessel. The funnel-and-beaker trap is used to prevent that.
Making glass syringe needles for glass syringes with Luer joints.
Here's a quick little run down of how to pull down your own long borosilicate glass needles for a typical Luer joint glass syringe using a Bunsen
burner or map gas torch.
Didymium glasses and a proper ventilation hood are strongly recommended if you will be doing this a lot.
1. Take some graphite tape, ceramic tape, or a folded slightly damp paper towel and stick it in between the two joints and twist them together to get
them a little stuck.
2. rotate the inner joint in one hand, and hold onto the end of the outer joint with tweezers in the other hand.
3. Move the outer joint back and forth through a hotter flame while consistently slowly but steadily rotating. Be sure to not twist the tube, and try
to rotate with your hands in synchrony.
4. When the tube is red hot and doesn't 'feel' solid, bring the tube out of the flame and begin pulling while slowly rotating at a consistent, steady
pace.
5. Let the pulled down joint cool down on a surface that won't burn.
6. Use a scoring knife, a sharp piece of tungsten, or the edge of a file, put a small 'score' on the tube where you want the end to be and pull it
off. Wetting the score helps, and be sure to pull and bend at the same time with the score facing away from you. Safety goggles are highly recommended
if you are doing this a lot. It doesn't take much pressure with such small tubing.
Here's a more comprehensive guide on scoring and splitting up glass tubing and rod.
http://www.ilpi.com/glassblowing/tutorial_cutting.html
7. You can give it a drip tip with a dremel and some kind of diamond bit wheel. Be sure to do that under running water, and with safety goggles.
8. If you would like you can touch the freshly scored end of the tube to the side of the flame to 'flame polish' it so it's less sharp. Be careful as
it does not take much, and it's very easy to close up the end of the tube.
9. To dislodge the two joints, use a small piece of wood to lightly tap around the outer joint while very lightly pulling the inner joint, or while
angling the two in such a way that the outer joint will slide off. You can also lightly tap the outer joint against a table while lightly pulling. Try
to pull by and bigger section of the outer joint left as opposed to pulling on the section that was pulled down to a smaller diameter.
HIGHLIGHTS FROM THREADS
Chemistry in general
Preparation of elemental phosphorus (The lesser known reactions)
Cleaned, boiled and dried chicken bones (bone meal can also be used with if used as organic fertilizer) are burned until they have turned into white
ash. 2g of this bone ash are mixed with 0.5g magnesium powder or activated carbon powder and 0.5g Diatomaceous earth. The mix is heated and the vapors
are lead underwater for the vapors to cool and condense to white phosphorous. ---low-temperature production of phosphorus, the most interesting
candidates appear to be phosphates of lead, bismuth, and antimony. silver phosphate can be reduced and yields finely divided metallic silver and
phosphoric acid, which is catalytically reduced in the presence of the silver to give free phosphorus.
Lead phosphate is particularly easily reduced under hydrogen or methane with hydrogen resulting in the highest yields and methane at 50%. The lead
phosphate is heated up to 300°C to drive off any existing water. Once the temp hits 300°C the hydrogen is turned on and the temperature slowly
raised to 500°C. The hydrogen reduces the lead phosphate by ripping off the oxygen molecules and forming lead phosphide. Upon the cessation of
evolution of water, the furnace is again slowly raised up to somewhere between 650-800°C. According to the patent, small amounts of phosphine are
liberated at around 600°C. This makes sense, the lead phosphide probably starts to break down somewhere around 600°C and thus liberates phosphine,
which subsequently start to be reduced to hydrogen and elemental phosphorus at around 650C, so basically at the beginning of the reduction temp the
phosphine being liberated is not hot enough to break down. phosphates can be reduced by hydrogen at temperatures between 300°C and 750°C.
Pyromorphite starts to react at 300°C and is completely reduced at 850°C.
Other possible reducing agents calcium carbide would make a good reducing agent in such a reduction as phosphate reduction. I suspect that sodium
polysulfide would work well in a phosphate reduction. Using sodium acetate as a carbon source fusing Hexasodium metaphosphate with Sodium acetate the
mixture liquefies quickly and a strong smell of garlic was released indicative of the presence of phosphourus. Sodium acetate acting as a carbon
source and a flux because once the melt was fluid there is a large release of Phosphorus.
Phosphorous from phosphine
phosphine with dimethylchloramine, which reacts to form elemental phosphorus and dimethylammonium chloride (as reported here: http://pubs.acs.org/doi/abs/10.1021/ic50067a009)
Organic Chemistry
Acetic Anhydride
Many amateurs have attempted to make acetic anhydride at home due to its tremendous usefulness in organic chemistry. Only a few have succeeded,
though a write-up of the preparation of acetic anhydride from sulfur, bromine, and anhydrous sodium acetate was reported by Magpie. Another more
accessible method involves the reaction of acetyl chloride and anhydrous sodium acetate.
A preparation that is as of yet not well-researched involves the dry vacuum distillation of anhydrous zinc acetate, which gives off acetic anhydride
and converts to basic zinc acetate. If achievable, this would mean a route to acetic anhydride that only requires glacial acetic acid and cheap zinc
metal.
LEGAL AND CHEMICAL AVAILABILITY ISSUES
Overall chemicals are becoming increasingly harder to aquire due to terrorism and chemophobia. There was great news last year for amature chemists in
Texas.
The law in Texas regarding laboratory glassware that if it is bought it must be registered was repealed in 2019.
FORUM MATTERS
Ever wonder how many members are in sciencemadness
We have 288131 mad scientist members.
Spam problem eliminated With the change of registration to email only (date start needed) sciencemadness is finally is not having problems with
constant spam alot of hard work was going into avoiding this last resort but the board has benefited from the change. With even a span killer
designated to take on the problem before and a subform named Test Forum with the Forum Moderator as streety It was a Test area for testing spam
prevention mechanisms.
The message everyone sees is the reasoning
The management apologizes for the inconvenience, but it is necessary to keep abusive users and bots out.
CHEMISTRY MEMES

PERSONAL DEVELOPMENTS
Tdep's great new (top secret) job doing chemistry for the military.
Cou's business venture of fragrance compounds
Metacelsus has completed his Master of Philosophy to become a PhD student and is working with Induced pluripotent stem cells.
Clear_horizons_glass business venture as a laboratory glassblowing service
Farewell/OBITUARY
Sadly over the last 12 months the Science Madness community lost two of our amazing members, Magpie and Melgar. Both Magpie and Melgar contributed
volumes to this forum as evidenced by the long and heart felt threads that were posted upon hearing of their passing.
Melgar
http://www.sciencemadness.org/talk/viewthread.php?tid=155324...
Magpie
http://www.sciencemadness.org/talk/viewthread.php?tid=154563...
Farewell, but may you ever live on in your invaluable contribution to Science Madness.
To quote Polverone, 'Magpie was one of our most long-established members and brightest lights. He will be sorely missed.'
The loss of Melgar at such a young age came to a huge shock to the community. He will always be remembered and always sadly missed.
References
Make Sulfuric Acid (Copper Sulfate Electrochemical Method)
https://m.youtube.com/watch?v=5dUSF9Gl0xE
Sulfuric acid water and sulfur (electrobromine process)
https://m.youtube.com/watch?v=6ms6xbPhdVs
Make Sulfuric Acid by the Copper Chloride Process
https://m.youtube.com/watch?v=l2AkVYxDSKc&t=13s
Make Sulfuric acid (metabisulfite/oxidizer method)
https://m.youtube.com/watch?v=okvvD3-DF9U
-------
Sulfur trioxide
http://www.sciencemadness.org/smwiki/index.php/Sulfur_trioxi...
Sulfur Trioxide and Oleum, by GARAGE CHEMIST [Publication]
http://www.sciencemadness.org/member_publications/index.html
--------
Ostwald style nitric production [Technochemistry]
http://www.sciencemadness.org/talk/viewthread.php?tid=71282
---------
pulling down capillary tubes
https://www.youtube.com/watch?v=2yKHvKCatmM
Preparation of elemental phosphorus [Prepublication/Chemistry in general]
http://www.sciencemadness.org/talk/viewthread.php?tid=65
Preparation of Acetic Anhydride
[Prepublication/Organic chemistry]
http://www.sciencemadness.org/talk/viewthread.php?tid=15021
--------
RECOGNITION OF COLLABERATION
J_sum1
B(a)P
Heptylene
Cou
clearly_not_atara
Texium (zts16)
Clear_horizons_glass
Metacelsus
[Edited on 26-11-2020 by symboom]
|
|
|
Clear_horizons_glass
Harmless

Posts: 42
Registered: 30-9-2019
Location: Pullman, WA
Member Is Offline
Mood: Glass
|
|
Very cool! 
Clear Horizons Laboratory Glassblowing Services
-------------------------------------------------------------
www.clearhorizonsglass.com
Phone and Fax:
(855) LAB-GLAS
(855) 522-4527
Have a glass project you want made? email me at
info@clearhorizonsglass.com
or message us here |
with a U2U |
message |
\/
|
|
|
Clear_horizons_glass
Harmless

Posts: 42
Registered: 30-9-2019
Location: Pullman, WA
Member Is Offline
Mood: Glass
|
|
Not sure if this is the kind of thing you are looking for or not, but I made this post just now about how to make glass syringe needles for glass
syringes with Luer joints. Feel free to put this in this report or the next one if you would like.
Here's a quick little run down of how to pull down your own long borosilicate glass needles for a typical Luer joint glass syringe using a Bunsen
burner or map gas torch.
Didymium glasses and a proper ventilation hood are strongly recommended if you will be doing this a lot.
1. Take some graphite tape, ceramic tape, or a folded slightly damp paper towel and stick it in between the two joints and twist them together to get
them a little stuck.
2. rotate the inner joint in one hand, and hold onto the end of the outer joint with tweezers in the other hand.
3. Move the outer joint back and forth through a hotter flame while consistently slowly but steadily rotating. Be sure to not twist the tube, and try
to rotate with your hands in synchrony.
4. When the tube is red hot and doesn't 'feel' solid, bring the tube out of the flame and begin pulling while slowly rotating at a consistent, steady
pace.
5. Let the pulled down joint cool down on a surface that won't burn.
6. Use a scoring knife, a sharp piece of tungsten, or the edge of a file, put a small 'score' on the tube where you want the end to be and pull it
off. Wetting the score helps, and be sure to pull and bend at the same time with the score facing away from you. Safety goggles are highly recommended
if you are doing this a lot. It doesn't take much pressure with such small tubing.
Here's a more comprehensive guide on scoring and splitting up glass tubing and rod.
http://www.ilpi.com/glassblowing/tutorial_cutting.html
7. You can give it a drip tip with a dremel and some kind of diamond bit wheel. Be sure to do that under running water, and with safety goggles.
8. If you would like you can touch the freshly scored end of the tube to the side of the flame to 'flame polish' it so it's less sharp. Be careful as
it does not take much, and it's very easy to close up the end of the tube.
9. To dislodge the two joints, use a small piece of wood to lightly tap around the outer joint while very lightly pulling the inner joint, or while
angling the two in such a way that the outer joint will slide off. You can also lightly tap the outer joint against a table while lightly pulling. Try
to pull by and bigger section of the outer joint left as opposed to pulling on the section that was pulled down to a smaller diameter.
Here's a video on pulling down capillary tubes:
https://www.youtube.com/watch?v=2yKHvKCatmM
Here's a link to where you can order Luer joints from Chemglass.
inner: https://chemglass.com/ground-joints-inner-luer
outer: https://chemglass.com/ground-joints-outer-luer
Clear Horizons Laboratory Glassblowing Services
-------------------------------------------------------------
www.clearhorizonsglass.com
Phone and Fax:
(855) LAB-GLAS
(855) 522-4527
Have a glass project you want made? email me at
info@clearhorizonsglass.com
or message us here |
with a U2U |
message |
\/
|
|
|
brubei
Hazard to Others
  
Posts: 187
Registered: 8-3-2015
Location: France
Member Is Offline
Mood: No Mood
|
|
nice page
sciencemadness reference links are broken
I'm French so excuse my language
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Cool summary! I'm actually back from the UK (I completed my MPhil in August 2019) and now I'm a PhD student living in the Boston area. Unfortunately I
don't have much time for home science anymore, since I'm spending almost all of my time on my PhD project. (In fact, I'm about to head to the lab
today to feed my iPSCs.)
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
The newsletter has been updated if there are any mistakes or changes needed let me know anyone is welcome to add to this collaborative project.
|
|
|
Texium
|
Thread Moved
5-12-2022 at 12:12 |
|