EverythingAl2O3
Hazard to Self
 
Posts: 51
Registered: 3-9-2019
Member Is Offline
|
|
More thoughts (rants) on AlCl3 Chlorination of alcohols
So I did more thinking since my previous post about why AlCl3 doesn't work for chlorinating aliphatic alcohols and this is what I came up with as
potential reasons and possible solutions to it.
So we know that AlCl3 can chlorinate benzyl alcohols on its own given the reaction the following reaction conditions "To a 100 mL round flask were
added 20 gram alcohol, 60mL of 1, 4-dioxane, and 1.75 equiv. of AlCl3. Then the flask was immersed in a preheated 70˚C oil bath for 5 h. "
I believe the reason for this is because the ring can break aromaticity to help kick off the OH+ and make room for the Cl- ion. I believe this would
be the primary mechanism because if Sn2 were the primary mechanism then aliphatic alcohols should react with it (along with no stereochemistry studies
that I can find to confirm chirality changes). And by extension if my hypothesis is correct then allyl alcohols should be capable of chlorinating in
this fashion with preferential chlorination on the less substituted carbons and tertiary alcohols should be able to chlorinate. If I'm correct about
the carbocation intermediate this could lead to allyl shifts in the case of primary substituted alkenes and secondary alcohols. Which also explains
why we don't see chlorination of primary aliphatic alcohols.
Now I think the issue with AlCl3 being the reactant for aliphatic alcohols is that we have an intermediate that is not stable given the inability of
the primary alcohol to leave we can have a carbocation, so we need to rely on Sn2, but if we try to make a chlorine leave the complex we lose the
negative charge stabilization making it much more likely that it will reverse back to the tetrahedral aluminum complex. I believe a solution to this
would be the addition of a chloride salt that is soluble in dioxane or possibly another polar aprotic solvent that will not interact with the AlCl3.
Possibly DMF, Sulfides, non-enolizable carbonyls, DMSO, and ethers.
I would like to know what anyone thinks about this. Am I just ranting about nothing? Is there evidence that I am absolutely wrong about this? Am I
possibly on to something here? Feel free to rip this apart


[Edited on 9-12-2020 by EverythingAl2O3]
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
There are way easier reagents to use for chlorination of alcohols than aluminum chloride. Have you ever worked with the stuff? I've tried making it a
couple times and it is a pain to hassle with. Fumes all over the place and absorbs moisture quicker than you can scratch your ass. The first time I
tried making it was inside via ZnCl2 reduction with aluminum and I thought I was doing something wrong after 15 minutes of nothing. Then bam! White
fumes started pouring out of every crevice of my glassware. Looked like someone set off a smoke bomb. I had a tube feeding from the take off adapter
to outdoors but it clogged up somewhere and blew the joints apart.
Long story short, there are easier reagents to work with.
(Edit) Check this one out!
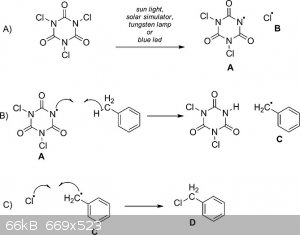
The reason the benzylic carbon is easily halogenated is because of resonance stabilization within the benzene ring. The charge can be transferred
throughout the ring. The benzylic carbon is so reactive I've seen people chlorinate toluene to benzyl chloride without any catalyst, just TCCA. I'm
sure you could do it with plain chlorine and a UV lamp. I would look up some already defined reactions before trying to reinvent the wheel.
Edit: posting proper reaction I was referring to
[Edited on 10-12-2020 by Opylation]
|
|
|
EverythingAl2O3
Hazard to Self
 
Posts: 51
Registered: 3-9-2019
Member Is Offline
|
|
| Quote: |
The first time I tried making it was inside via ZnCl2 reduction with aluminum and I thought I was doing something wrong after 15 minutes of nothing.
Then bam! White fumes started pouring out of every crevice of my glassware. Looked like someone set off a smoke bomb. I had a tube feeding from the
take off adapter to outdoors but it clogged up somewhere and blew the joints apart.
|
Yeah I'm just gonna buy some AlCl3, If I want to synthesize it for fun I'd probably use a method like the one below.
| Quote: |
I would look up some already defined reactions before trying to reinvent the wheel
|
I already have and I am planning on performing them particularly with TCT, but also want to explore the extent that AlCl3 can be used. I'm all about
Al chemistry.
Attachment: Microscale Preparation of AICI3.pdf (1MB)
This file has been downloaded 237 times
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I think the reason you see chlorination with benzylol is just due to the stability of benzyl carbocation and the steric hindrance of the phenyl ring.
This means that the AlCl3-BnOH complex can release Bn+ instead of H+. With a tertiary alcohol this likely just gives an alkene, while benzyl or allyl
cations cannot beta-eliminate and react with chloride. Primary cations never form and secondary cations likely form too slowly (Al(OiPr)3 is stable).
I would not expect this rxn to generalize in any way. Sn2 depends on the nucleophilicity of the halide substituent, but aluminium halides' presence
decreases the nucleophilicity of halide ions. With benzyl alcohols you get a rxn due to the Goldilocks reactivity of benzylium: reactive enough to
attack haloaluminate anions but stable enough to form in the first place.
[Edited on 9-12-2020 by clearly_not_atara]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
To whom it may concern:
TCT is not equal to TCCA (unfortunately) !!! :-)
But there's surely someone on SM who has experience with TCT as I recall.
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
Quote: Originally posted by Pumukli  | To whom it may concern:
TCT is not equal to TCCA (unfortunately) !!! :-)
But there's surely someone on SM who has experience with TCT as I recall.
|
Whoops! I meant to find a reaction referring to TCCA chlorination and I guess I went a little fast and misread what reaction I grabbed. I did mean to
reference TCCA in my text but the image I posted is not the reactions I was referring to. Good catch!
|
|
|