| Pages:
1
2
3
..
8 |
RustyShackleford
Hazard to Others
  
Posts: 195
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Violuric acid salts (fantastic colors and variety)
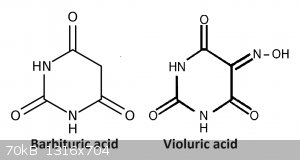
Violuric acid can be prepared from barbituric acid (via NaNO2) or from alloxan (via hydroxylamine chloride). Barbituric acid can be prepared from
condensation of diethyl malonate and urea, but i have not tried this, i simply purchased the barbituric (from S3 chemicals).
To make the violuric acid i followed the preparation on illumina from barbituric acid: https://illumina-chemie.de/viewtopic.php?t=5502
"
Procedure:
Sodium violurate:
6.40 g (50 mmol) of barbituric acid were dissolved in 100 ml of hot water in a beaker. A solution of 3.80 g (55 mmol) of sodium nitrite in 10 ml of
water was added to the hot solution, and it immediately turned deep purple. A large amount of sodium violurate precipitated when it cooled down. To
complete the reaction and precipitation, 10 g of sodium chloride (because of the addition of equal ions) as well as 1 g of sodium acetate and 3 ml of
acetic acid were added to bring the pH to approx. 4-5 (the reaction is de facto complete in the pH range) and stirred for about 1-2 hours at room
temperature. The pH was then brought back to the weakly basic range (lowest solubility) with 2.5 g of NaOH and allowed to crystallize in the
refrigerator for a few hours.
The product was suction filtered and washed with a little cold water and air dried for a few days.
Yield: 9.85 g of sodium violurate dihydrate (84.5% of theory)
violuric acid, from sodium violurate:
7.8 g (33.5 mmol) of sodium violurate dihydrate were suspended in 20 ml of water and 10 ml of conc. Hydrochloric acid added. The deep violet
suspension turned brown-pink in color. It was stirred well for about 1 hour at room temperature, suction filtered and washed thoroughly with dilute
HCl, the color becoming a little lighter (cream-colored). The product was air dried for a few days.
Yield: 5.70 g of violuric acid monohydrate (88.2% of theory)
Note: if you touch the damp precipitate with a metal spatula, blue-violet spots will appear at the point of contact after a short time. Obviously,
even the smallest traces of metal lead to a salt / complex formation."
I found that i got 86% yield on converting barbituric acid to violuric acid.
Most of the salts themselves were produced by simply heating the acid with the corresponding base in water and evaporating dry, but some (like barium
and ethylene diamine) were prepared by adding salts of them to the solution of sodium violurate left behind in the preparation of the acid.
So far i have made: Na, K, Fe, Cu, Ba, ethylene diamine, hexamine, Sn, NH4, Mn, Zn and creatinine.
im planning to make: Sr, Ca, Pb, Al, aniline, benzylamine and quinoline
Let me know if there are particular salts youd want to see, so far it has made great colors with everything i have tried



My favorites are these: Ethylene diamine, Mn and hexamine (bottom to top)

as well as the copper one.

The iron one isnt bad either, has a rich dark blue color in solution, but solid is so dark its almost black, not very fun

|
|
|
ShotBored
Hazard to Others
  
Posts: 124
Registered: 19-5-2017
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Those colors are awesome, dude. Only iron compound I've ever seen blue like that is Prussian blue. I'd be interested in seeing Pb, Sr, or guanidine
salts! Or anything further on this subject really. These colors are astounding.
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 195
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Quote: Originally posted by ShotBored  | | Those colors are awesome, dude. Only iron compound I've ever seen blue like that is Prussian blue. I'd be interested in seeing Pb, Sr, or guanidine
salts! Or anything further on this subject really. These colors are astounding. |
Pb and Sr are in the works, guanidine i do not currently have, but maybe some day. maybe put a thing in your calendar to check back in a week or two,
ill update the post as i produce new salts.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Gorgeous! I'm interested in seeing the nickel complex.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
ShotBored
Hazard to Others
  
Posts: 124
Registered: 19-5-2017
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by RustyShackleford  |
Pb and Sr are in the works, guanidine i do not currently have, but maybe some day. maybe put a thing in your calendar to check back in a week or two,
ill update the post as i produce new salts. |
Derp for me, reading comprehension helps since you clearly state which ones you are working on above...oops.
Other compounds I would think would have interesting colors would include Cr and Mn...and As, though I usually stay away from As compounds myself.
Hydrazinium might have vibrant colors too.
Finally, I did a lot of work with Nickel coordination chemistry in college. I always thought Nickel compounds had incredibly beautiful hues, mostly
greens and deep reds are what I saw.
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 195
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Quote: Originally posted by ShotBored  |
Other compounds I would think would have interesting colors would include Cr and Mn...and As, though I usually stay away from As compounds myself.
Hydrazinium might have vibrant colors too. |
Mn i already did lol  . Cr probably not hard, hydrazine might also be possible,
but if i could make it from the sulfate that would be best, the freebase is kind of a pain to deal with and make pure. . Cr probably not hard, hydrazine might also be possible,
but if i could make it from the sulfate that would be best, the freebase is kind of a pain to deal with and make pure.
Ni i would certainly do, just need to get some.
I basically want to make the violurate salt of every cation i have or can reasonably get
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Nice! Differences in colours of Na, K and NH4 salts are quite unusual. I would like to see Co2+ salt, cobalt salts in general have very nice colours.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Rustyshackleford, I did a load of work on these compounds years ago and posted a few microphotographs of the the crystals. Most M+ and M2+ metals
form amazing coloured salts. They were the subject of much research back in the early 1900s because of the tendency of the colourless violuric acid to
form strongly coloured slat with ions that normally form white salts. There are a few interesting exception Fe2+ forms a deep blue complex and cobalt
and orange one. The shape of the crystals under the microscope is also highly characteristic. I used to use these reactions to identify alkali and
alkaline earth metals in easily soluble minerals and Mn oxides.
The barium and Strontium salts are quite stunning under the microscope.
http://www.sciencemadness.org/talk/viewthread.php?tid=14644&...
I will have to dig out the other photos from a back-up disc. I even tried to encourage a thread dedicated to this type of microchemical test but to no
avail.
I also starting preparing barbituric acid derivatives N-methyl, N,N'-dimethyl, thio- etc. and also related isonitroso-isoxazoles. Interesting to see
that someone else is finally interested in these most unusual salts.
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 195
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Quote: Originally posted by Boffis  | | @Rustyshackleford, I did a load of work on these compounds years ago and posted a few microphotographs of the the crystals. |
Thats very cool! with the amazing colors this acid produces im suprised its not more widely known about.
Do you have any tips for me in preparing these salts or the derivatives of the acid?
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Quote: Originally posted by Bedlasky  | | Nice! Differences in colours of Na, K and NH4 salts are quite unusual. I would like to see Co2+ salt, cobalt salts in general have very nice colours.
|
Different colours for the alkali metals is special indeed!
@RustyShackleford What about Caesium?
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Thanks for the post! I will be making some 
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Well, nice!
What about ammonium? Or silver? Cadmium? Mercury 2+?
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
This is really remarkable. I missed this thread up to now, but what most strikes me is the different colors for different alkali metals and ammonium.
I do not know any other anion, which gives such striking different colors when e.g. Na(+) is exchanged with K(+).
It's also interesting to see Ba(2+) vs. Sr(2+) vs. Ca(2+). Usually, salts of these three anions also have more or less the same color.
I do have diethyl malonate. Up to now I did not have any real use for it, I obtained it as part of a package of old chemicals, years ago, and it still
is in its unopened bottle. Now I have a use for that 
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
How about caesium as the cation? If you don't have any I could send you send you some CsOH for shipping costs. In what part of the world are you
located?
[Edited on 17-12-2020 by Tsjerk]
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 195
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Quote: Originally posted by Tsjerk  | How about caesium as the cation? If you don't have any I could send you send you some CsOH for shipping costs. In what part of the world are you
located?
[Edited on 17-12-2020 by Tsjerk] |
i sent you a U2U, hopefully we can work something out!
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Incredible colors! I'm adding this compound to my todo list
Rubidium would be cool to see as well if you are going for cesium!
Also, if you have an interesting results with Al3+, maybe the rare earth would be interesting to investigate. They usually all react the same way and
form pale-colored compounds, so a difference from this would be noteworthy.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
The colours you see can vary a lot even with a single cation depending on the hydrate and the ratio of acid to cation, pH, temperature etc. There are
a whole series of papers on these salts by Hantzsch and others from 1909-1910. I hhave translated some of them but I can't find them just at the
moment. Caesium forms a pure blue salt under warm neutral conditions. I spent a lot of time looking at organic buffers to try and find one that
doesn't form a coloured salt with violuric acid. In the end the best was anion exchange resin beads washed with NaOH then acetic acid. Lithium is
another interesting one, normally a bright red salt but then occasionally an insane fushia pink! Mn, Zn and some other divalent transition cations
also react, but Cu2+, Fe2+ and Co react to produce soluble coloured complexes that are hard to crystallise. Interestingly UO2++ also reacts to form
intensely yellow blades. I orginally examined this reagent as a microchemcial reagent for investigating secondary uranium minerals and Mn oxides both
easily soluble in dilute acetic acid (the best medium for salt formation)
|
|
|
valeg96
Hazard to Others
  
Posts: 253
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
What about the lanthanides? I'm also curious about unusual metals such as U, Th and the likes.
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 195
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
I myself do not have any pure lanthanides nor U or Th. I only have the mixture from dissolving lighter flints. If you have lanthanides or U/Th , i
could send you some violuric acid, as long as you promise to post pics in this thread 
|
|
|
itsallgoodjames
Hazard to Others
  
Posts: 276
Registered: 31-8-2020
Location: America Lite
Member Is Offline
|
|
I wonder what violurate esters might look like. Also, metal complexes as the cation might be interesting. There's always so many possibilities with
interesting ions.
Nuclear physics is neat. It's a shame it's so regulated...
Now that I think about it, that's probably a good thing. Still annoying though.
|
|
|
valeg96
Hazard to Others
  
Posts: 253
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
Out of curiosity, can you post a full procedure for the preparation of the metal salts?
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 195
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
i just measured stoichiometric metal base and violuric acid by mass, heated together in water solution and evaporated dry with heat. wasnt really
anything advanced. I was doing 2-3 at a time.
|
|
|
valeg96
Hazard to Others
  
Posts: 253
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
Is that a proven procedure, backed up by literature? Where do the anions go? What do you mean with "metal base"?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by valeg96  | | Is that a proven procedure, backed up by literature? Where do the anions go? What do you mean with "metal base"? |
A metal base would be a metal carbonate or a hydroxide. Do you need us to explain to you where the anions go when you react an acid with a metal
hydroxide or carbonate?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
valeg96
Hazard to Others
  
Posts: 253
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
Oh, well, it makes sense then. I'm just not used to call CuCO3 or Cu(OH)2 a "metal base", and I believe I've never heard anyone call transition metal
hydroxides or carbonates "bases".
You're called DraconicAcid but you're caustic as hell today, bruh.
|
|
|
| Pages:
1
2
3
..
8 |