vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Gallium (II) iodide
Today I made Gallium (II) iodide. It is a green solid. I tried this particular method:
Ga + HgI2 = Hg + GaI2
I did not try to separate the compound, I just wondered if it was real. However, to some extent the compound will be contaminated with mercury. I
think it is an interesting and simple reaction.

|
|
|
Metallophile
Hazard to Self
 
Posts: 82
Registered: 23-3-2018
Member Is Offline
Mood: No Mood
|
|
Wikipedia says this is a mixed valence compound of Ga(I) and Ga(III). This page on "GaI" is pretty interesting:
https://en.wikipedia.org/wiki/Solid_%22GaI%22_Precursor
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
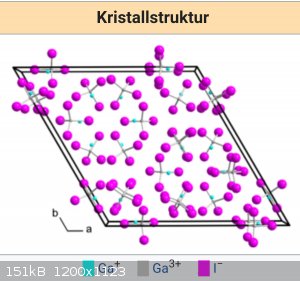
Yes it is a mixed compound. Gallium 1 and 2 iodides two of them are green. Look at crystal structure. But i like this reaction, because its simple and
this compound is very nice and interesting.
|
|
|