| Pages:
1
2 |
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
There are many "strange" bonds like three electron bonds, three-centered-two electron bond, four-centered-two electron bond, bonds in aromatic and
homoaromatic compounds etc.
In the case of these radicals, boranes, strange cations (like H2+, He2+, CH5+...) etc. is good to draw MO diagram - you can see number of unpaired
electrons, you can calculate bond order etc.
Btw. NO have also dimer N2O2 - both are in equilibrium in liquid state.
https://en.wikipedia.org/wiki/Nitric_oxide
https://en.wikipedia.org/wiki/Dinitrogen_dioxide
[Edited on 10-1-2021 by Bedlasky]
|
|
|
Plinius
Harmless

Posts: 4
Registered: 11-1-2021
Member Is Offline
|
|
I have been an occasional visitor for some time but I found this thread so interesting that I decided to register an account.
About 30 years ago I worked at a company which built a plant for production of nitric oxide from sulfur dioxide and nitric acid. The plant was made of
glass, in principle large scale laboratory equipment. The raw nitric oxide was dried by washing it with concentrated sulfuric acid in a packed column.
The plant started up without problems. After a few days a surprised operator discovered that the sulfuric acid in the storage flask under the drying
column had developed a beautiful violet colour. I was assigned to the task finding out why the acid had turned violet. The natural starting point was
looking in Gmelin where the answer was easily found.
The plant was very colorful during production. The reaction column was brown due to the nitrogen dioxide and the sulfuric acid purple. On top of that
the cooling water contained an anti-freeze which had some flourescein added to it. Visitors were impressed.
As a by product dilute sulfuric acid containing nitric acid was obtained. During the design phase I wondered how that acid solution would be disposed
of in en environmentally acceptable way. The local management found a creative solution to the waste disposal problem. They donated the acid solution
to a local pig farmer who added it to the manure produced by the pigs instead of purchased acid. The addition of acid decreased the smell (stench is
probably a better word) from the manure. A win-win situation.
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Plinius: Welcome to the forum! I never thought that such a rare compound can be produce during industrial operation.
|
|
|
Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Plinius, that is very cool! I would love to see that plant in operation.
I have in the meanwhile found the answer to the questions on what the copper now actually does. It turns out it is something quite different, not a
salt of the purple acid. Rather, the purple acid just serves as a donor of NO. In strong sulfuric acid, copper will form an addition compound with NO,
denoted by Gmelin with CuSO4 · NO. This is indicated by the fact that it can be made from pure NO and copper sulfate in strong sulfuric
acid, and its absorption spectrum has nothing in common with the one from purple acid. It is water sensitive and hydrolyses below 70% it seems.
Another nail in the coffin is that copper chloride actually forms the blue color too, giving CuCl2 · NO, and its absorption spectrum is
the same. It would be interesting to try this, dissolving anhydrous CuCl2 in alcohol or acetone, then passing NO into it. I assume this is
or at least is related to the blue complex that woelen obtained in the experiment linked earlier.
The source is Gmelins handbook of inorganic chemistry copper part B-1 page 579:

[Edited on 19-1-2021 by Diachrynic]
we apologize for the inconvenience
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Diachrynic: I found paper about reaction of Cu(0), Cu+ and Cu2+ with NO in strong acids. End product of these reactions is [Cu(NO)]2+ complex, which
you mentioned in your post.
Cu + 3NO + 2H+ --> [Cu(NO)]2+ + N2O + H2O
2Cu + 4NO + 4H+ --> 2[Cu(NO)]2+ + N2 + 2H2O
2Cu+ + 4NO + 2H+ --> 2[Cu(NO)]2+ + N2O + H2O
Cu2+ + NO <--> [Cu(NO)]2+
https://sci-hub.se/https://www.journal.csj.jp/doi/abs/10.124...
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Gents the attached extracted from an old book discusses the "violet acid".
The book is "Lecture Experiments in Chemistry by Fowles G" which can be downloaded here
https://b-ok.global/book/3164501/d69c55
which in my opinion is a very interesting and informative book especially for chemistry amateurs like me.
Attachment: Violet acid.pdf (2.7MB)
This file has been downloaded 293 times
|
|
|
Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Bedlasky, that is a great find! That is exactly what I was looking for and confirms the text from Gmelin but in more detail. Thank
you!
Lion850, thanks for this contribution as well! The more sources mention the compound the more weird it seems I (and many others that
I asked) had literally never heard of it before I randomly stumbled over it. The formula given is slightly different but not too far off from the
modern interpretations.
Here are three more relevant papers:
Wright, Wu, Hayton, 2010, "Structural Characterization of a Copper Nitrosyl Complex with a {CuNO}10 Configuration", doi:
10.1021/ja105930b
Fraser, 1961, "The dissociation of nitric oxide-copper(II) halide complexes in alcohols. The preparation of the copper(II) fluoride nitrosyl",
doi: 10.1016/0022-1902(61)80150-4
Fraser, Dasent, 1960, "The Composition of the Nitric Oxide Complexes of Cupric Halides", doi: 10.1021/ja01487a022
The last one seems very relevant for the experiment woelen performed. I assume halides can stabilize the blue complex so it doesn't decompose as fast
in water and without it strong sulfuric acid is needed as the solvent.
This is a very interesting tangent from the purple acid. The paper Bedlasky found by Tsumori and Xu plus the Gmelin entry demonstrate that copper
actually decomposes the purple acid and just using its NO to form a much more strongly colored complex itself. This means that no copper is the way to
go if actual purple acid is desired. Still, a very interesting experiment in itself.
By the way, purple acid is supposed to turn cherry red when strongly cooled, most often dry ice bath temperatures are mentioned, but I am unsure
whether or not is is a sharp or a gradual transition, although I assume the latter. This might be a neat addition to the preparation of purple acid if
dry ice is available.
we apologize for the inconvenience
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
This is a fun and relatively easy experiment! I can confirm the section of the Atomistry article which says the substance becomes red on cooling.
After chilling in a dry ice/acetone bath for a few minutes, the blue-purple color changed to a deep cherry red. When cooled further, the mixture
mostly solidified into a red slush. The purple color returned upon warming.
Purple Acid:

Low-temperature Red Chromomorph:

Solidification:

The section from the Fowles book mentioned earlier is really interesting, especially the part describing the catalytic cycle in the lead chamber
process. If I had the capacity I'd try out the whole range of demonstrations.
Lead Chamber Cycle
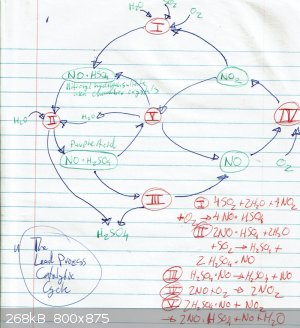
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Nice red colour! Does Atomistry say at what temperature change in colour occur?
|
|
|
Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Wow mayko, that's awesome! I never had dry ice to test this. Thank you for documenting it!
we apologize for the inconvenience
|
|
|
Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Translation of Gmelin pages complete
Well, I forgot about it somewhat, but since I was reminded of this compound again, I decided to finish where I left off two years ago and translated
the Gmelin pages.
It's by far the most complete account on purple acid/N2O2HSO4 I have found so far.
Attachment: purple_acid_translated.pdf (359kB)
This file has been downloaded 117 times
[EDIT] I am reworking the wording somewhat as I noticed some clunky phrases in the beginning especially, an updated version should be done soon.
[EDIT2] New version added.
[Edited on 21-1-2023 by Diachrynic]
we apologize for the inconvenience
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
That's great! Thank you for translation, your work is really appreciated!
|
|
|
Neal
Hazard to Others
  
Posts: 116
Registered: 24-12-2021
Location: Chicago, IL, USA.
Member Is Offline
|
|
Thermochromism: where chemicals change color on temperature. My inorganic textbook lists very few examples.
|
|
|
j_sum1
Administrator
       
Posts: 6219
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Link no longer works. But this sounds like a fantastic book.
I will have to dig up a copy.
|
|
|
j_sum1
Administrator
       
Posts: 6219
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
And here it is.
Lecture Experiments in Chemistry by Fowles G
https://ia601705.us.archive.org/26/items/dli.ernet.476101/47...
|
|
|
Neal
Hazard to Others
  
Posts: 116
Registered: 24-12-2021
Location: Chicago, IL, USA.
Member Is Offline
|
|
So I'm looking at
https://www.sciencemadness.org/smwiki/index.php/Purple_acid
And it's mp and bp are both decomposes? So what does it do at room temperature, decompose to both a solid and a gas at the same time?
|
|
|
Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Thanks for the link Neal, I will have to update the page, since it mainly quotes literature that is out of date.
Purple acid has not been made in the pure solid state. Unless its sulfuric acid solution is stored under high pressure NO gas, it decomposes in
solution to NO and NOHSO4, as it is in equilibrium with them. Read the translated Gmelin pages on the details, the constant of this
equilibrium has been measured (although I haven't seeked out the values yet).
[Edited on 24-1-2023 by Diachrynic]
we apologize for the inconvenience
|
|
|
| Pages:
1
2 |