vanBassum
Hazard to Self
 
Posts: 50
Registered: 16-4-2019
Member Is Offline
|
|
Measuring the perchlorate cell
Hello,
I have been playing around with a PbO2 perchlorate cell for a little while now. While doing so I was wondering if the voltage of the cell could be
used to determine when the process is done. This motivated me to build a power supply capable of logging the voltage and current. I also added a
temperature probe, so I can keep track of the relation between current and temperature. I tried to find a current that corresponds to a cell
temperature of 70C witch turns out to be around 13A. The cell is positioned outdoors, so I suspect to see slight influences by the weather, although
the weather needs to change considerably to be of any influence since the cell is positioned in a bucket to protect it from rain etc.
The voltage is measured at the output of the power supply, obviously the voltage at the cell is lower, but I don't think it matters that much. I am
more interested in the change of voltage when the process runs to completion. I might change the supply in the future to accept sense lines. Let's see
where this measurement gets me first.
The cell was started with NaClO3 with probably some NaCl contaminate. I had just enough NaClO3 to fill the cell ones, so I won't be topping of the
cell with more NaClO3, but I will be adding water to make sure the electrodes stay fully submerged. Unfortunately my chlorate cell just died, I have
ordered the parts to build a replacement, but that will take some time to do.
Now, I would like to be able to preform some quantitative tests to keep track of the production of perchlorate. I could do this by hand, but I am kind
of lazy  However I don't think an automated way is possible for me. However I don't think an automated way is possible for me.
Oh, I can also program the power supply to turn of, or to a very low current when the voltage gets above a certain threshold. This could protect the
electrodes from running to long. I have read that PbO2 can be run to completion, but for MMO this might be a nice addition.
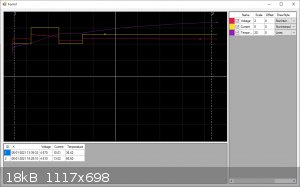
[Edited on 6-1-2021 by vanBassum]
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 473
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
the perchlorate production totally depends upon current and you wanna lower the temp to 45 by water cooled bath.
current densities of 200 to 225ma/cm^2 are good for this purpose.
voltage doesnt have to be high as I usually run 4.7 to 5.5 volts depending on how good my electrical connections are and wire length which can affect
cell voltage compared to supply voltage.
my cell runs at 16 amps.
I measure my current using calibrated inline shunt but all my runs were cursed by stainless steel thermometer probe erroding and releasing the
chromates!!
Overall though an additional note for PbO2 electrodes on Ti is if they do fk up its easy to repair via re plating them or using superglue to first
create a polymer-PbO2 or graphite substrate onto the bare Ti before plating in membrane plating cell.
My electrodes havent failed yet and are holding on very well.
[Edited on 7-1-2021 by mysteriusbhoice]
|
|
|
vanBassum
Hazard to Self
 
Posts: 50
Registered: 16-4-2019
Member Is Offline
|
|
I just put the cell in a water bath, since the cell was already placed in a bucket this was quite an easy fix  I do have an aquarium pump, so I might circulate the water in the bucket to a much larger reservoir for continuously
cooling. I do have an aquarium pump, so I might circulate the water in the bucket to a much larger reservoir for continuously
cooling.
About the current density, the anode is sandwiched between 2 cathodes and is 150x60mm, so 150 * 60 = 9000 mm^2 = 90 cm^2. But I have 2 sides so this
will be 180 cm^2 effectively. 13000 mA / 180 cm^2 = 72 ma/cm^2. That is quite low compared to the 200 ma/cm^2 you are proposing. I don't know if this
is a problem or if it just takes more time to complete. My PSU maxes out at 20A, that corresponds to a current density of 111 mA/cm^2. Still a bit
low, I could use 2 psu's, both powering one half of the anode and one cathode, but I would have to buy this. (And this project is getting quite
expensive already  ) I hope to avoid this and just wait longer for the process
to finish, otherwise I'd have to buy a second supply. ) I hope to avoid this and just wait longer for the process
to finish, otherwise I'd have to buy a second supply.
I also measured the voltage directly at the connections of the cell. This is 4.15V while the PSU delivers 4.85V. Now I can calculate the resistance of
the wires, 4.85 - 4.15 = 0.7V > 0.7V/13A = 53.84mOhms. Not bad if I say so myself. Then again the wires are 4 mm^2 if I remember correctly.
About fixing the cell, I have thought about this when my MMO died, Perhaps I can convert it to a PbO2 cell, although this would require messing around
with dissolved lead salts, and I am not quite sure if this is something I like to be playing with too much. Can it be done with lead anodes or do you
need PbO2 specifically? Anyway If I decide to go this route I should do some research.
By the way, I've added another screenshot,
Between cursor 1 and 2: I topped of the cell with water.
Between cursor 2 and 3: I added water to the bucket, so the cell is in a water bath.
Quite a dramatic drop in temperature and still going down, Now I can crank up the current 
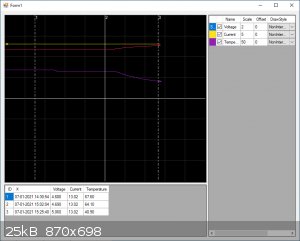
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 473
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
how on earth did your MMO die it literally is supposed to last 20 years continuos run!!
mine is over 8 years old and is going strong well maybe cuz I always run pH controlled using CaCl2 buffer and HCl during near end of run to replenish
CaCl2.
dead MMO means you can convert it into a PbO2 electrode so dead MMO is never a waste for me if it ever happens to me!
Pb(ClO4)2 or Pb(ClO3)2 is what I use to make PbO2 electrodes and its fairly easy to prepare these salts using ion exchange electrolysis.
The dead MMO electrode can be stripped and sanded down and you can just get some graphite powder or PbO2 powder from batteries and superglue to make a
conductive substrate.
You then put this electrode into your ceramic membrane plating cell which is the same cell you made your lead salts in.via ion exchange electrolysis.
run for 1 day then swap out electrodes with lead anode to replenish lead perchlorate.
using lead chlorate leads to the decomposition reaction on the anode side to form ClO2 so use sodium perchlorate as feed in order to form lead
perchlorate.
[Edited on 7-1-2021 by mysteriusbhoice]
|
|
|
vanBassum
Hazard to Self
 
Posts: 50
Registered: 16-4-2019
Member Is Offline
|
|
Good to know that my MMO can be used for a new purpose. I don't really trust the state the MMO was when it came in. I suspect it was a cut off from
something that was already used extensively. Then again It might have been my fault for letting it run to long. I did let it run continuously and just
top off the cell with fresh NaCl solution. Perhaps there was a point when I was too late adding more chloride and the MMO got destroyed. I lack a bit
of experience I find it difficult to tell how far along the process is. A well, this is a great learning experience for me 
Quite cool to see the voltage increase when the cell cooled down, from 64.1C @ 4.69V to 36.3C @ 5.12V
BTW, I upped the current to 15A, the temperature is 35C. I will let this run for a few hours to see where the temperature will settle.
|
|
|
vanBassum
Hazard to Self
 
Posts: 50
Registered: 16-4-2019
Member Is Offline
|
|
I just took a sample of the cell to which I added saturated KClO3 solution. A precipitate of KClO4 formed in the test tube, so things are working.
 But I also notice that the electrolyte turns brownish, Is that normal? I would
think it is wear on the anode? Also, How can I measure how much chlorate is left in the cell and how do I know when the process is finished? But I also notice that the electrolyte turns brownish, Is that normal? I would
think it is wear on the anode? Also, How can I measure how much chlorate is left in the cell and how do I know when the process is finished?
http://www.chlorates.exrockets.com/anodes.html
This site claims that a PbO2 cell can be left running until no more chlorate is present. So I shouldn't be a problem to just let it run.
Also, I kind of doubt the claim 'no chlorate'. I suspect that a sensitive test like the indigo carmine test still test positive after the cell has
been run to completion. That's no problem, chlorates can easily be destroyed with some HCl.
[Edited on 8-1-2021 by vanBassum]
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 473
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
Quote: Originally posted by vanBassum  | I just took a sample of the cell to which I added saturated KClO3 solution. A precipitate of KClO4 formed in the test tube, so things are working.
 But I also notice that the electrolyte turns brownish, Is that normal? I would
think it is wear on the anode? Also, How can I measure how much chlorate is left in the cell and how do I know when the process is finished? But I also notice that the electrolyte turns brownish, Is that normal? I would
think it is wear on the anode? Also, How can I measure how much chlorate is left in the cell and how do I know when the process is finished?
http://www.chlorates.exrockets.com/anodes.html
This site claims that a PbO2 cell can be left running until no more chlorate is present. So I shouldn't be a problem to just let it run.
Also, I kind of doubt the claim 'no chlorate'. I suspect that a sensitive test like the indigo carmine test still test positive after the cell has
been run to completion. That's no problem, chlorates can easily be destroyed with some HCl.
[Edited on 8-1-2021 by vanBassum] |
Yes the brown is due to chloride contamination in your starting material.
I measure remaining chlorate by taking a small amount of liquor and adding HCl then heating in test tube to see if yellow appears ClO2.
Yes PbO2 can run to completion and my homemade electrodes have done 1 run to completion although its quite inneficient but I say its worth it but it
can run to no chlorate because it will then start to evolve ozone gas and this is infact the other use for PbO2 electrodes.
The runtime of a full conversion run though is extremely extremely long like 5x the run time which is fine for me since I just pump it with all the
amps.
To maintain electrodes after many long runs I plan to just plate PbO2 again for a few hours to restore the coating.
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 473
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
Quote: Originally posted by vanBassum  | Good to know that my MMO can be used for a new purpose. I don't really trust the state the MMO was when it came in. I suspect it was a cut off from
something that was already used extensively. Then again It might have been my fault for letting it run to long. I did let it run continuously and just
top off the cell with fresh NaCl solution. Perhaps there was a point when I was too late adding more chloride and the MMO got destroyed. I lack a bit
of experience I find it difficult to tell how far along the process is. A well, this is a great learning experience for me 
Quite cool to see the voltage increase when the cell cooled down, from 64.1C @ 4.69V to 36.3C @ 5.12V
BTW, I upped the current to 15A, the temperature is 35C. I will let this run for a few hours to see where the temperature will settle.
|
What I do is use runtime calculations to first estimate how much chlorate I want to produce.
I then keep adding salt every day and keep chloride levels well above 100g/l.
I also make sure pH is controlled and measured periodically by boiling down a small sample and adding water and pH indictator paper is then dipped to
see the pH.
The end solution will contain high amounts of chloride but its quite easy to seperate NaCl from NaClO3 using a gas stove and fractional
crystalization.
also when running chlorates keep temps above 60 Celsius.
|
|
|
vanBassum
Hazard to Self
 
Posts: 50
Registered: 16-4-2019
Member Is Offline
|
|
Thanks for the information!
I am currently building a new MMO cell to replace the old one. When I start that cell up, I'll make sure to not run the cell to long. As you say,
NaCl and NaClO3 are quite easy to separate. I just use a non-stick wok pan, the non-stick layer holds up perfectly.
For the perchlorate, I'm not sure yet if I want to run to completion. Perchlorate can be separated with acetone and any residual chlorate can be
destroyed with hydrochloric acid. It's a bit more effort, but it isn't that much extra work anyway.
I've included another screenshot of the progress, you can see when I refreshed the water bath by the temperature dip. BTW, the spikes in the
temperature are false readings, this has something to do with my measurement system. I haven't got around to fixing this yet. It doesn't influence the
electrolysis anyway.
BTW, I have ordered the ingredients to build a second supply, so I can monitor the chlorate cell as well.
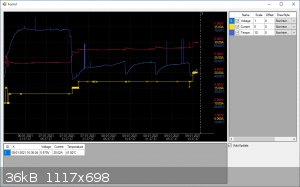
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 473
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
btw about running 100% conversion it is that the PbO2 anodes seem to errode a little bit a 2nd time when chlorate levels get low.
The errosion is again minimal but its present and if you have no way to re plate your electrode after dozens of runs then a 100% conversion run is not
viable for you.
I have to do it because acetone where im in is not cheap for some reason because its out of stock in the local hardware.
I however can easily plate more PbO2 again if I need to replenish the coating if it gets thin which probably wont be any time soon anyway.
|
|
|
vanBassum
Hazard to Self
 
Posts: 50
Registered: 16-4-2019
Member Is Offline
|
|
I finished up the run yesterday. When testing with HCl it tests negative for chlorate, but the indigo carmine test still tests positive for chlorate
as I suspected. There was a very fluffy suspension of brown stuff, although that was very little and easily filtered off. The filtrate was clear like
water. Last steps left, destroying any remaining chlorate with HCl, boiling of remaining acid and drying the final product. I do have to say, not
having to go through the hassle of separating with acetone is very nice. I haven't decided yet if I let the cell run to completion in the future.
The yield so far is about 1 litre of saturated solution, so more or less 1 kg from a single run.   
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 473
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
yea im attempting to do a 100% conversion run or atleast as far as it goes because I can easily repair my electrode if funny stuff happens during the
end.
but the last time I did a near 100% conversion with solution only slightly turning yellow it took 4 days to run and probably because I have no
additives in my cell because I cant get persulfate.
im gonna run the thing for 6 days though now at day 3 it is starting to smell like ozone but there is still some chlorate in the cell with HCl test.
the perchlorate I have now will most likely be used to make ammonium perchlorate after all chlorate is destroyed and for precaution I plan to expose
the solution to UVC just to drive off any chlorate that may still linger after producing ammonium perchlorate or perchloric acid or maybe some more
exotic perchlorates.
overall I have 1kg of perchlorate from the previous run and for this run its gonna be another 1kg.
|
|
|
vanBassum
Hazard to Self
 
Posts: 50
Registered: 16-4-2019
Member Is Offline
|
|
Ohh I just destroyed another MMO, aaargh. Let it run for too long, I should program in a stop when the current starts to decline. Ah man, now I need
to go shopping for another MMO.
The perchlorate cell has produced quite some perchlorate by now. I've just started the 3rd run. I did notice that the electrolyte is quite brown, and
I can clearly see a deposit of presumable PbO2 on the titanium cathodes. I'm not sure if this is normal or if my cell is wearing extensively.
I am actually considering skipping MMO all together and just start with chloride, that would make things easier, although it would mean more wear on
the PbO2.
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 473
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
how is it that you keep killing your MMO when its best to just keep adding NaCl even towards the end and also keeping pH controlled and temps high at
65 Celsius.
seperation of NaCl and NaClO3 is super easy I always just make sure to have more than 25% NaCl by wt as safety leverage and it will be forwardfed
anyway
|
|
|
vanBassum
Hazard to Self
 
Posts: 50
Registered: 16-4-2019
Member Is Offline
|
|
Yea I know, I let it run for a couple of days, then I do a fractional crystallization. The filtrate gets saturated with chloride, and then it goes
back into the cell. Then I top it of with fresh NaCl solution.
I just let it run too long and the voltage rose too much. As you can see in the included graph, the purple line is the voltage where I compensated for
the drop in the wires. As you can see, the current started to drop, and I started the cell on fresh NaCl. No forward fed filtrate, just plain NaCl in
water. As you can see, in the beginning the current started to rise, so I had some hope but after a while it was clear that the electrodes were done.
Sad as it is, nothing to do about it now. I did however start experimenting with making my own PbO2 electrodes. If I get that working then I will stop
with MMO and only use lead. Although I never had much luck with electroplating anything, so I doubt PbO2 will turn out decent enough. But one can at
least try of course, at least it's a learning experience. Although a bit of an expensive one, MMO isn't too easy to find for me. Every once in a while
they pop up on eBay.
It also was the last piece of MMO I had laying about, I still have some smaller pieces but if I decide to keep on going with the MMO I'll search for a
new sheet online.
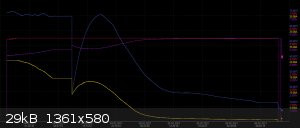
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 473
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
I always add chloride into the cell every few days to prevent any of this from happening.
when I do fractional crystalization I actually boil it down once then after letting it cool up to -10C I boil it down again to half it volume and the
crystals that come out at boiling point are NaCl which gets fed back into the cell and the liquid is then frozen again and this step ensures its
impossible to run the cell for too long because what gets fed back into the cell is 90% NaCl crystals with a bit of NaClO3 soaked in.
the solution is boiled down even further until like 100ml is left and this is either forwardfed to the next run or precipitated with KCl.
as for your voltage friggin 6v tho..
I never run my chlorate cell more than 5v usually from 4.3 to 4.7 volts and perchlorate from 4.8 to 5.5 volts.
Also pH control in chlorate cell preserves your electrodes and its easy using CaCl2 buffer + HCl where due to the CaCl2 buffer it means you only need
to add some HCl once every 2 days which means once every run on day 2 to react with Ca(OH)2 to form CaCl2 again.
|
|
|
vanBassum
Hazard to Self
 
Posts: 50
Registered: 16-4-2019
Member Is Offline
|
|
So, I am trying to create my own PbO2 anodes. I've used a dead MMO as cathode and 2 lead anodes on each side. The electrolyte is just plain tap-water
with a bit of sulfuric acid. I let this run for a while at 1A and the lead anodes formed a black layer, presumable PbO2. The old MMO didn't change, or
at least not visibly to the naked eye. I added some NaClO3 to the cell. The idea being that the lead anodes are essentially converted PbO2 anodes.
Hoping the following would happen:
1. The NaClO3 gets converted to NaClO4.
2. Somehow the PbO2 gets deposited on the old MMO.
After a couple of days I can see the MMO turning the same color as the store bought PbO2 anode. So I am hopeful that this works. I will let this go
for another couple of days to see where this gets me. If this turns out to be working ill skip the MMO entirely. Then I don't care about the wear on
the anodes anymore since its easy and cheap to fix. As you can see, I am not a chemist and I don't have the skills to read and decipher academic
papers. There is probably a lot of information out there about PbO2 electrodes. But I like to try these things, although I mostly stay away from the
really toxic stuff since I don't have the right tools and a lab to do this safely. The PbO2 cell is pushing it already, it's outside, so that's less
risk of me dying horribly, but there is still the issue of cleaning everything when the experiment is done. My idea is to slowly letting everything
evaporate and sealing the solids in some ziplock bags. I will use labels, so I won't forget what's in there if a few years go by. Eventually I think I
would have to pay some company to take care of it.
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 473
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
thing is even if that works perchlorate cells run at insane current densities to even get decent efficiency like around 180 to 200 ma/cm^2 which yes
ive also tried lead anodes but sadly they dont work and turn to powder when pushed that hard even when the surface is polymer impregnated.
overall better to make lead chlorate and use a membrane plating bath but yea you gotta deal with highly toxic soluble lead salts!!
|
|
|
vanBassum
Hazard to Self
 
Posts: 50
Registered: 16-4-2019
Member Is Offline
|
|
Oh I think you missed the point, I am trying to create a PbO2 anode from my old MMO. There will probably be some perchlorate production as a side
product. But the main goal is to revive the anode.
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 473
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
Btw if you wanna do something to atleast make it so the lead waste is stored in a good manner is to precipitate most of it out with sodium bicarbonate
or sodium phosphate then just evaporate down the water by leaving it out somewhere DONT HEAT; then once it becomes a slurry you can store that slurry
or put it in a membrane cell cathode chamber to plate out solid lead at low current density or lead sponge.
Also PbO2 anode from dead MMO is good and will work nicely because it actually will restore the conductivity of the shit MMO and you just have to use
MMO as a cathode for awhile before anodically plating it.
this anode for use from chloride to perchlorate needs a really thick PbO2 coating and I suggest doing alpha-beta plating where you first plate alpha
PbO2 in lead acetate thin coats only needed for alpha then you plate beta PbO2 using lead chlorate/perchlorate do something like 20mins in the acetate
and 5 hours in the perchlotate bath repeat for thicker coating and I also prepare acetate from membrane electrolysis and sodium acetate feed.
coating useful for use in chloride to perchlorate must atleast be 0.5mm to 1mm thickness which thickness can be known by initially weighing the
electrode say for eg 25 grams then weighing it after sometime for instance 75 grams.
you can then obtain the weight added which is 50 grams then divide by the density of PbO2 9.38 then that value you get in volume is divided by the
toal surface area of the anode to get thickness because LWH/LW = H.
|
|
|