reactofurnace
Hazard to Self
 
Posts: 76
Registered: 17-7-2015
Member Is Offline
Mood: Volatile
|
|
Mechanism help. (Acetanilide)
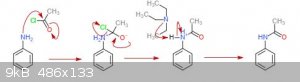
Hey guys. I'm having trouble settling on a correct mechanism for the reaction between acetyl chloride and aniline to form acetanilide. Is a base
required? I saw one mechanism use a base and one with a proton transfer, which confuses the heck out of me.
Sidenote: I believe this reaction is just for the illustrative purpose of an assignment; we must also explain why it's not an appropriate route to
making acetanilide.
Here is the mechanism I'm leaning towards attached.
Thanks for any help given 
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
For this reaction, you really have two options. Either you don't use triethylamine, and create hydrogen chloride. The HCl then reacts with the
aniline, which means that you need an excess of aniline. Or, if you want to conserve aniline, you can add a stronger base like triethylamine which
takes the proton like you showed in your mechanism and liberates a chloride anion. Hope this helps 
Edit: If you want to make diagrams like that, instead of copy them from chemistry stack exchange  , use this website : https://web.chemdoodle.com/demos/2d-sketcher , use this website : https://web.chemdoodle.com/demos/2d-sketcher
[Edited on 5-2-2021 by Triflic Acid]
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Oh, Acetic Anhydride might work better. No HCl generation.
Aspirin might also be able to perform the Acetylation. Ummm, though possibly that is a method used to acetylate amino-acids. I'd better review that.
Pyridine might also be an HCl scavenger for a reaction like that. It can form a Hydrochloride, but it cannot be acetylated itself.
Pyridine smells like rancid butt.... In a bad way! Easy to make though.
Also possible to make amides of that type by producing the Aniline Acetate salt, followed by heating to bring about dehydration. Possible to use
Ketene too. A more industrial approach.
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Well, on second thought. That might not be such a bad approach.
You could react Acetyl Chloride with Anhydrous Sodium Acetate, in glacial acetic acid. Making Acetic Anhydride. Solves your base problem.
You could then react with Aniline, producing the Acetanilide.
Do the whole thing in glacial acetic acid. Add water, the Acetanilide drops out of solution, the by-product is NaCl.
Not ideal, but the problem becomes workable.
|
|
|