Laboratory of Liptakov
International Hazard
    
Posts: 1335
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
Ba(ClO4)2 primary for ETN...?
Substance Ba (ClO4) 2 was tested with hexamine. It appears that a 75:25 mixture of BaP + HMTA has initiating effects on ETN. The substance itself is
not able to pierce the steel sheet. But it is capable of detonation. Which can be seen on the plate. The estimated VoD is 1500 - 2500 m / s. But mixed
1: 1 with ETN will cause ETN to fully detonate. In the steel cavity wall 1mm, diameter 6 mm. Prepare: BaP and HMTA are dissolved on an inert surface
in a small amount of water. It is then dried at 80-100 Celsius. The resulting mixture has very strong energy properties. The individual dry grains
burn so violently that they essentially explode on the heated aluminum foil. Individual small explosions resemble an electrical short circuit. Both
green color and sound. It is a very funny substance for home experiments. 250mg in aluminum foil detonates when heated by a candle. Ba (ClO4) 2 was
prepared from excess BaO2 to dilute HClO4.
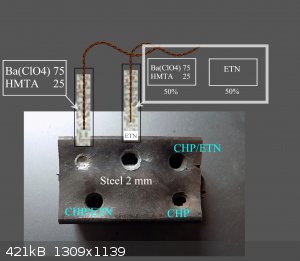
[Edited on 9-4-2021 by Laboratory of Liptakov]
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
Elemental Phosphorus
Hazard to Others
  
Posts: 184
Registered: 11-11-2016
Location: Is everything
Member Is Offline
Mood: No Mood
|
|
Very cool! You always seem to be creating new energetic mixtures. I would maybe be a bit worried about the stability of the mixture in storage, does
it seem to be storage-stable?
Also, why did you use barium peroxide to prepare the compound, it seems like it should be easier to prepare from some basic barium salt like barium
hydroxide or barium carbonate and perchloric acid, did you just happen to have some?
All in all though, seems like a very cool invention, to make a cheap primary that doesn't need a booster, as long as it isn't too sensitive. How's the
shock sensitivity?
Very cool invention as always!
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Isn't barium perchlorate exceedingly deliquescent? I seem to remember it was sold as a drying agent. That wouldn't be a good characteristic for an
energetic material.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1335
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
1) Storage stability mixture 75/25 not tested yet. Yes were available only BaO2. Sensibility on anvil/hammer very low, and only in Alu foil. Without
alu foil is almost impossible get blow. Medium sesibility on flame. Similarly a like perchlorate flash I estimate.
2) Yes, is hygroscopic, simlarly a like NaNO3. But much less, than NH4NO3. After 12 h on metal plate in wet garage 7 Celsius, slightly wet grain.
After short dry process 5 minute at 80 Celsius dry grain again. No serious problem yet. A new mixture will of course examinated. Different ratios and
etc.
Unfortunately, next attempts shows, that substance is not reliable for DDT. Next 2 tests were failed. Any way, Ba(ClO4)2 is interesting oxidizer for
very good green flame, especially with hexamine.
[Edited on 8-4-2021 by Laboratory of Liptakov]
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Fantastic LL, especially the etn-free version that made quite a dent! Interesting to ask if KCLO4 version is possible to make, I suspect that it will
not be ignitable, but in that case some metal oxide catalyst usually can help. Something like KCLO4/hex/CuO (Cr2O3,MnO2,Fe2O3,etc.) maybe worth to try
(or even KCLO3  ) )
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1335
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
Substance 75:25 is not reliable. It would require deeper research, maybe the addition of aluminum or something. In any case the CHP works reliably.
And his base is perchlorates also...
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
JohnDoe13
Harmless

Posts: 21
Registered: 3-2-2017
Member Is Offline
Mood: No Mood
|
|
Have you tried to bind the BaP / HMTA with DBSP or high grade NC?
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1335
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
It seems that things are different. For the first, functional experiment, Ba (ClO4) 2 was used, but neutralized with a large excess of ammonia water.
So a diamine, or tetraamine perchlorate of Barium, was formed. (DBaP?) And it has the right DDT properties. By comparison, TACP always precipitates in
ammonia water. But DBaP is completely soluble in ammonia water. When burning grain, no difference is seen in the open air. But in the closure, the
properties change dramatically. While Ba (ClO4) 2 + HMTA 75:25 is not able to start ETN, DBaP + HMTA 75:25 does. The experiment was repeated with
success. That's all well and good, but why bother with the production of Barium salts, when a much stronger and completely reliable CHP66 is
available. Which is TACP + 6% HMTA + 6% NH4ClO4. The new DBaP substance does not provide any advantage. In frame DDT. The Research of DBaP was stop.
[Edited on 9-4-2021 by Laboratory of Liptakov]
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
Elemental Phosphorus
Hazard to Others
  
Posts: 184
Registered: 11-11-2016
Location: Is everything
Member Is Offline
Mood: No Mood
|
|
Well while you may not be very interested in the compound that you obtained, it seems like an interesting piece of new research. I have never heard of
tetraamine or diamine barium salts, and searching ChemSpider didn't give any matches either. While CHP may be a better mixture, I am still interested,
and barium salts aren't particularly expensive or rare.
I should be getting some barium nitrate in the mail soon, and maybe I can try out this experiment.
Was the compound prepared by mixing barium perchlorate solution with hexamine dissolved in ammonia?
Anyway, it seems you have created a new energetic mixture. Even if it's not more practical than CHP detonators, it's definitely cool. 
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1335
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
Thanks. I have no idea what exactly came about. I'm just guessing there's some double salt. Interestingly, Ba (ClO4) 2 dissolved in 1:20 ammonium
water dissolves completely, but nothing precipitates. Neither within 12 hours nor during cooling to zero Celsius. After evaporation of the liquid, the
granules thus treated have no energy properties. But after mixing with hexamine they get very strong. And they burn at much higher temperature than
CHP. Alu foil is instanty melted, almost evaporated. Alu foil from CHP is without damage. Only thin film elementar copper.
Ba(ClO4)2 0,75g (from ammonia water) as dry powder was mixed with 0,25g dry hexamine. After were added 3 - 4 drop ammonia water 25%. Arised porridge
consistency. Warm air was used for drying process during a mixing. Until to consistency wet grain. Sieve 1mm was used for final grain. Temperature of
inert surface about 50 - 80 C. Dry time 10 minute. Done.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
MineMan
National Hazard
   
Posts: 998
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Laboratory of Liptakov  | Substance Ba (ClO4) 2 was tested with hexamine. It appears that a 75:25 mixture of BaP + HMTA has initiating effects on ETN. The substance itself is
not able to pierce the steel sheet. But it is capable of detonation. Which can be seen on the plate. The estimated VoD is 1500 - 2500 m / s. But mixed
1: 1 with ETN will cause ETN to fully detonate. In the steel cavity wall 1mm, diameter 6 mm. Prepare: BaP and HMTA are dissolved on an inert surface
in a small amount of water. It is then dried at 80-100 Celsius. The resulting mixture has very strong energy properties. The individual dry grains
burn so violently that they essentially explode on the heated aluminum foil. Individual small explosions resemble an electrical short circuit. Both
green color and sound. It is a very funny substance for home experiments. 250mg in aluminum foil detonates when heated by a candle. Ba (ClO4) 2 was
prepared from excess BaO2 to dilute HClO4.
[Edited on 9-4-2021 by Laboratory of Liptakov] |
Great discovery!
Why not mix Al in? Could ddt without etn, which molecular explosive should be avoided
|
|
|