Monoamine
Hazard to Others
  
Posts: 160
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Avoiding over nitration (or generally over-electrophilic substitution)
I'm interested in making the para nitro benzoic acid. The generally accepted reaction is below, but I'm somewhat concerned that it might nitrate the
ring at the ortho positions too. I certainly don't accidentally want to make some kind of explosive!!
So basically I was hoping to ask, whether there is a risk of sticking on more nitros than wanted, or if there is a reason why only the ortho product
is obtained....?
Attachment: Reaction (191kB)
This file has been downloaded 170 times
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Use only a small molar excess of nitric acid that should limit multiple nitration as will lower temperatures. Lower temperatures will also reduce
oxidation of the alcohol group.
|
|
|
Monoamine
Hazard to Others
  
Posts: 160
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Quote: Originally posted by Boffis  | | Use only a small molar excess of nitric acid that should limit multiple nitration as will lower temperatures. Lower temperatures will also reduce
oxidation of the alcohol group. |
Thank you for the answer!
I looked at the mechanism, and apparently in general one gets a 50/50% (depending where you look) of the 2-nitro and the 4-nitro product.
Do you know how such a mixture is usually separated?
It's not obvious to me how you might separate the two, since (I'm guessing) their solubility properties are the same, and they can't be distilled off
either since their boiling points are ginormous.
Oxidizing the OH and then Protecting the ortho positions through sulfonation at the meta positions won't work either (see attached reaction scheme).
So this basically leaves column chromatography, but even that might be hard since they are probably also similarly polar...
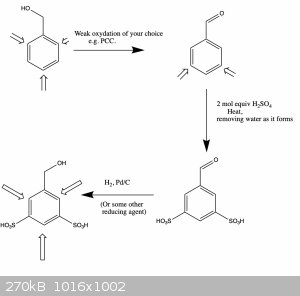
|
|
|
Monoamine
Hazard to Others
  
Posts: 160
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Purification procedure.
Ok, after some research I think that the best way to separate the ortho and the meta product is by freeze distillation:
The ortho product melts at ~ 69-72oC
The para product melts at ~ 92-94oC
I guess the way to do this would be to allow everything to come to room temperature so it "freezes". Wash away and neutralize any remaining acid,
etc...
Slowly heat the solids to about 72oC, and drain off the liquid portion. Then increase the temperature to about 95oC and collect
the rest.
Probably wanna wash it a few more times to make sure all the acid is gone.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I suspect that plenty of para will dissolve in the melted ortho.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Why don't you start from toluene? You can crystallize part of the p-nitrotoluene at -15 C
Then you can oxidize it to p-nitrobenzoic acid by refluxing with water solution of KMnO4
|
|
|
Monoamine
Hazard to Others
  
Posts: 160
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
I see. So maybe I'm thinking about this the wrong way around...
Maybe it would be better to melt the two products in a solvent in which they are not soluble. (Although I'm guessing that this would form two
layers...
Then the solution is cooled very very slowly so that the para product can gently solidify.
Ideally, the solvent used should also be less dense than the liquid nitro compounds, so that any solid ortho product will precipitate to the bottom.
At this point the top molten ortho product is syphoned off and the solids in the solvent layer filtered off.
The ortho product can now simply be left to cool until it solidifies.
For extra purity, this procedure should probably be repeated a few times...
|
|
|