Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hydrazone or triazole?
I have prepared some Dimethylglyoxal mono-oxime (2,3-butanedione mono-oxime) via Vogel's route to dimethyl glyoxime (the dioxime) but acidified the
reaction mixture instead of adding hydroxylamine to generate the dioxime. The resulting monoxime is easily recovered and purified. I have been
investigating the numerous condensation products of this dione-mono-oxime with hydrazine derivatives and aromatic amines etc.
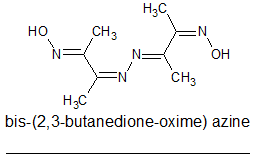
When I attempted to carry out the double condensation with hydrazine I got inconsistent results. I can find no reference to such an azine but the
azine contains 4 conjugate double bonds so is like to be yellow coloured.
The first time I tried it using 1.8g of Dimethylglyoxal mono-oxime and half a molar equivalence of hydrazine hydrate in hot methanol I got a bright
lemon yellow product composed of tiny thick tablets. The HH was added to the methanol solution of the mono-oxime in 3ml of methanol and heated in a
small beaker with a small water filled basin on top to act as a reflux condenser. The temperature was about 60 C for about 90 minutes and then left to
stand overnight. The lemon yellow product was much as expected.
In the next experiment I wanted to speed up the reaction so I used 3g of the mono oxime in isopropanol and refluxed the mixture for 20 minutes and
cooled the almost colourless solution. Masses of colourless needles formed and no yellow product. Thinking that I had added too much HH and that this
was the simple hydrazone I added more mono-oxime and refluxed again. On cooling the same colourless compound formed (the mono-oxime is too soluble in
alcohols to crystallise).
I repeated the initial experiment using methanol and heating to about 45-50 C for about 2 hours and again the yellow product formed. In both
experiments 1 and 3 the yield was almost exactly 50%.
The evidence seems to suggest that on refluxing at the boiling point of isopropanol the reaction follows a different course but to what? Once formed
the white silky fibrous product appears quite stables.
Looking at the structure of the initial butane-dione oxime hydrazone I wondered if the hydrazone and oxime moieties could react to loose water and for
4,5-dimethyl-1,2,3-triazole?
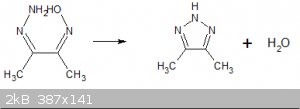
Is such a condensation possible? I have done a lot of reading on 1,2,3-triazole in the past and not seen a similar oxime-hydrazone condensation.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Have you tried starting from ketazine (by condensing the MEK with hydrazine) and doing the reaction with nitrites second? Seems like it could work.
With your product, was it explosive? Seems pretty nitrogen rich. Also, given that I just made some ketazine yesterday and still haven't got around to
hydrolyzing it to hydrazine sulfate, I might try to make this myself. Please send some photos as well, a lemon yellow product sounds interesting
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Was about to start the mono-oxime prep, only to find that all my isopropyl nitrite decomposed. Ah well, time to make a new batch
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Okay, hit a slight problem. How do I freebase the sodium salt of the mono oxamine?
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Considering the analogy to the formation of furazans from bisoximes I would say the formation of triazoles from oxime-hydrazones is not otherworldly.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Triflic Acid  | | Have you tried starting from ketazine (by condensing the MEK with hydrazine) and doing the reaction with nitrites second? Seems like it could work.
With your product, was it explosive? Seems pretty nitrogen rich. Also, given that I just made some ketazine yesterday and still haven't got around to
hydrolyzing it to hydrazine sulfate, I might try to make this myself. Please send some photos as well, a lemon yellow product sounds interesting
|
Hi Triflic; I was investigating the properties of the mono-oxime and its formation of hydrazones seemed like an obvious first choice for examination.
The idea of preparing the azine-dioxime from MEK ketazine is an interesting one, I would be interested to hear how you get one.
When you talk about freebasing the sodium salt are you talking about the sodium salt of dione-mono-oxime? If so I used 30%HCl added in small portions
to the ether extracted solution that had been previously chilled to about 0 C. Vogel recommends not allowing the temperature to rise above 15 C. It
took me 3 goes to complete the neutralisation and I had to filter off the liberated base after the second cycle because the liquor had become almost
unstirrable. I then recrystallised it from water with some charcoal to remove the brown tar and colour.
Hi Sigmatropic; I am running out of lab time but when I get back next time I am going to try and prepare a larger batch of the white material and then
try permanganate/KOH oxidation on it to see if I can prepare 1,2,3-triazole-4,5-dicarboxylic acid from it as I already have this compound prepared
from benzotriazole to compair it with.
I'll take some pic next time!
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Are there any non-acid catalysts for first step of the reaction. I feel like the acid catalyst would break down the methyl ethyl ketazine before
attacking the nitrite. Also, what was the pH at which you stopped adding the HCl? Neutral or acid?
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Ok, well freebased it. Going to try to get permission to run an nmr on the product to see whether or not It condenses.

There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Damn it
Was cooling down the flask, only to have the beaker flip over in the ice bath and spill. There goes the last two days of work.
[Edited on 30-6-2021 by Triflic Acid]

There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi Triflic, I'm a bit confused, which procedure are you trying out above? The reaction of an alkyl nitrite with ketazine with HCl as catalyst?
That's a very unfortunate accident, I've done this myself a few times. I tend to forget that as the ice melts the beaker becomes more buoyant.
In some procedures with half aromatic ketones like propiophenone sodium methoxide/ethoxide is used as the catalyst to condense alkyl nitrites. But
Vogel uses a small amount of conc HCl about 3ml per mole of nitrite. I guess it depends how rapidly the azine hydroxylises.
 Here's a photo of the lemon yellow bis(2,3-butadione-oxime)-azine for comparison. Here's a photo of the lemon yellow bis(2,3-butadione-oxime)-azine for comparison.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
@Boffis, I'm trying to react the ketazine with the alkyl nitrite. The nurdrage prep for converting the azine to ketone calls for 80 C for a few hours,
so I am hoping that it will not hydrolyze too rapidly. However, when I can get my hands on sodium metal, I'll be sure to try the methoxide catalyst.
Also, how much water did you recrystallize the mono oxime from? I'm going to have to put off those experiments until my hydrazine sulfate comes, since
my old ketazine is very impure and I feel guilty about using gallons of bleach during a pandemic.
[Edited on 1-7-2021 by Triflic Acid]
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi Triflic, I have found that you need about 2.2 to 2.5ml of water per gram of crude solid but it doesn't all dissolve, adding more water reduces the
yield further. It appears that the butadione mono-oxime polymerises or otherwise decomposes on prolonged heating in water to an insoluble product. I
have found the procedure below to be the best one providing that you have a vacuum filtration unit; the addition of a little charcoal or a mixture of
charcoal and kieselguhr helps achieve a white product.
For each gram of crude (light brown crystals) place 2.5ml of water in a beaker and heat to 80 C. Remove from the hotplate and stir in the mono-oxime
along with about 0.03g of charcoal per gram of crude oxime. Stir for 2-3 minute vacuum filter hot into a preheated Buchner flask. Pour into a
beaker/basin and allow to cool to room temperature and then chill in the fridge for 12 hours at about 4 C before filtering off the almost colourless
scaly crystals. From 42.08g of crude material I used 120ml of water, which proved to be too much, I recovered 24.75g of white crystals. The filtrate
can't be evaporated down at atmospheric pressure without considerable decomposition of the oxime to a white insoluble product. It is best to treat the
residue with hydroxylamine to precipitate dimethylglyoxime or hydrazine free base to give the azine.
The product crystallises slowly making it easy to filter the hot saturated solution provided that you use a vacuum unit. Also, its a good idea to keep
back a few crystals of the crude material to seed the filtrate as it tend to supersaturate then suddenly solidify. Ad a few seed crystals while the
solution is still warm.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Sad thing is, I don't have a fridge to cool down the flask. I guess I'll put it into a cooler filled with ice and leave it, but I don't know if that
will work. Is 12 hours strictly nessacary, or can it be for a shorter time?
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi Triflic, the mono-oxime generally crystallises slowly so the longer the time and the slower the rate of cooling the better the product and yield.
On one occasion no crystals formed before I put it in the fridge by the following morning still no crystals had formed so I stirred it with a spatula
and the whole lot solidified within 5 seconds but even with a Buchner funnel and vacuum I could separate very little liquid. Hence the advice to seed
the cooling solution but even then crystallisation can be slow.
The temperature/solubility curve of the mono-oxime in water is very steep so the difference between RT (about 18 C at present) and 4 C represents a
significant loss of product. In winter RT in my lab can be about 2-4 C most of Jan and Feb so the fridge isn't required  . .
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Well, this just keeps getting worse. A flask distilling ethylene glycol bumped explosively and sprayed everywhere. Especially on top of the hydrazine
sulfate I left to dry. Looks like I’m buying hydrazine sulfate then.
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
How did you convert hydrazine sulfate to alcoholic hydrazine?
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Btw,here is a photo of the monooximine. Didn’t bother to recryst.

There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi Triflic, yep that looks about the right colour for the crude oxime. If you don't make the solution too concentrated you can treat the aqueous
solution with a like charcoal and then add hydrazine sulphate and sodium carbonate solution. The azine should ppt as a yellow powder on warming gently
for a few hours. I used a methanol solution and commercial 98% hydrazine hydrate.
Are you going to have another go at the ketazine+hydroxylamine route?
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Yeah. Going to do that. Also, where did you buy your hydrazine hydrate? I was going to do an experiment on the different rnx conditions. If the
oximine is added dropwise to the hydrazine, the triazole should form since it has a 1:1 ratio of hydrazine to oxime. If the conditions are reversed,
with the hydrazine added to the oximine, as it did in your experiments, it gives the expected result of the azine, with a 1:2 hydrazine to oxime
ratio. And as for the ketazine hydroxylamine route, still working on that.
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I have had another go on a slightly larger scale (5g butadione monoxime) at condensing molar quantities of butadione monoxime and hydrazine hydrate. I
refluxed the two compounds for an hour in aqueous isopropanol and cooled. The white crystals formed were filtered off and washed with a little water,
the filtrate was treated with a little more hydrazine hydrate and refluxed for another half an hour to give a little more, slightly yellowish product.
I haven't measure the Mp of the product yet but will do on my return to the UK. Pechmann and Bauer (1) prepared the 4,5-dimethyl-1,2,3-triazole by
deamination of the 2-amino derivative and give the Mp as 97 C for the air-dried product and 70 C for the oven dried product. This suggests that the
initial crystals are a hydrate.
I tried oxidizing 3g with this product with alkaline potassium permanganate and obtained only 0.65g of white crystals that have still to be
investigated but these was a lot of evolution of nitrogen in the early part of the oxidation and only about half the theoretical amount of
permanganate was required which doesn't bode well for the product being 1,2,3-triazole-4,5-dicarboxylic acid. In the Pechamnn paper referred to above
the amino-triazole was prepared by oxidative cyclization of butadione bis(benzoylhydrazone) (he calls it dibenzoyl-diacetylosazone) and then
hydrolysis of the N-benzoyl amino-triazole.
Incidentally I also found a paper containing an example of the formation of a 1,2,3-triazole derivative by the cyclization of diacetyl oxime
phenylhydrazone (2)
1) Pechmann & Bauer, Berichte der C., p659-274 [1909]
2) Pechmann, Annalen, v262, p278 [1891]
|
|
|