RustyShackleford
Hazard to Others
  
Posts: 195
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Preparation of 3-cyanopropanoic acid from MSG (TCCA oxidative decarboxylation)
After seeing Bofis 2014 thread on TCCA oxidation of alpha-aminoacids to nitriles, i knew i had to try it : https://www.sciencemadness.org/whisper/viewthread.php?tid=29...
The substrate i had on hand was MSG, so i went with that.
I used a modified variant of the procedure found in “An Insight of the Reactions of Amines with Trichloroisocyanuric Acid” (file included at end
of post)
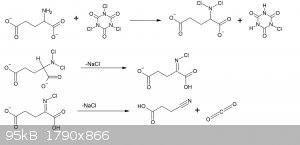
20g of MSG monohydrate (107mmol), 4.28g NaOH (107mmol) were dissolved in 40ml of water in a 250ml beaker. The solution was placed in an ice bath with
a thermometer and stirbar.

17.4g of powdered 96% TCCA (72mmol) was slowly spooned in, keeping the temperature below 30C. During the first 2/3rds of TCCA a vigorious evolution of
CO2 took place with every addition, ventilation is important. Addition took about 20 minutes.

After the TCCA had all been added, the solution was stirred for 10 minutes in the ice bath, after which the ice bath was removed and solution stirred
for a further 30 minutes.
The solution now at room temperature, was vacuum filtered to remove the excess cyanuric acid and unreacted TCCA. The powder was washed with 30ml
water, and then 30ml of ethyl acetate.
The filtrate was then then, with stirring, acidified with 3ml 30% HCl, and an excess of NaCl added as to saturate the solution. The point of
acidifying it after the filtration is to avoid unnecessary formation of chlorine gas.
The solution at this stage is slightly yellow from dissolved TCCA reacting with the HCl, so a spatula tip of metabisulfite is added to neutralize
this.
The solution is filtered again to remove the solid salt, salt is washed with 10ml water and then 10ml ethyl acetate into a separatory flask. The flask
is shaken well, and the ethyl acetate layer collected. The aqueous layer is further extracted with 3x25ml ethyl acetate.

Ethyl acetate is evaporated with airflow and stirring on the hotplate set at 60C. After about 2/3rds of the ethyl acetate had evaporated, there was a
buildup of solid in the beaker, this is likely residue from the water which was still in the ethyl acetate, so the remaining solvent is poured into a
clean beaker and further evaporated untill no volume change was observed.

The fluid was heated to 100C to drive off remaining solvent, raising the temperature turned it yellow, would be better to remove the last bit of
solvent with vacuum.
The beaker was placed into a CaCl2 dessicator overnight to cool, solidify and further dry from water.


After drying as much as it would in the CaCl2 dessicator, the product was titrated with hydroxide.
The titration gave 1.85mmol result of a sample that by mass (assuming 100% purity) would be 2.02mmol. This result is however still good because it
confirms we dont just have starting material or succinic acid(hydrolysis product of the nitrile). The product will ofc contain some impurity, but
seemingly not a huge amount.

The very hydroscopic product was transferred to a vial and weighed.
6.15g (58%) of 3-cyanopropanoic acid as waxy slightly wet light yellow solid.

If one has access to cheap alanine (NOT BETA), i think this reaction could be quite viable to produce acetonitrile.
Possibly one could distill it right from the reaction mixture, after neutralizing the residual TCCA. If anyone on this forum has alanine and time to
try this, i would greatly appreciate an attempt 
Cyanide also seems quite possible by reaction with glycine, but i believe this would be tremendously dangerous due to the cyanide being formed as HCN
gas
Attachment: deluca2004 (1).pdf (162kB)
This file has been downloaded 252 times
[Edited on 7-7-2021 by RustyShackleford]
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Can you get a melting point just to check? Possibly after recrystallization.
The literature value is 49.5–51 °C https://pubs.acs.org/doi/abs/10.1021/jo9804386
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi Rusty, Nice work and a good write up. I have tried this synthesis using the method of Hiegel et al 2004 (attached). I tried it at the same scale as
used in the paper and got a reasonable results considering the difficulty of vacuum distillation of very small quantities of the methyl ester. I then
tried recovering the free acid as you have done but only managed to obtain a small amount which after recrystallisation gave me only about 0.5g of
glassy fusible crystals ie <10% so you have done well. I will have to try again!
I also tried the same method on aspartic acid in the hope of obtaining cyanoacetic acid but I could not recover any cyanoacetic acid. I didn't try the
ester route. My theory was that cyanoacetic acid is too soluble in water.
I've attached Hiegel's paper for comparison. Note that they have got the name wrong, their glutaric acid is obviously glutamic acid.
Attachment: Conversion of a-amino acids to nitriles with TCCA Synth Comm Hiegel et al 2004.pdf (192kB)
This file has been downloaded 279 times
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 195
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
I just tested and got a melting point of 40-42C. I believe this is due to moisture, as the acid is EXTREMELY hydroscopic. it turns to a puddle in open
air in less than a minute. If you have other thoughts feel free to share, the only other substance i believe this could be would be succinic
semialdehyde, so i might try an aldehyde test on it.
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 195
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Quote: Originally posted by Boffis  | Hi Rusty, Nice work and a good write up. I have tried this synthesis using the method of Hiegel et al 2004 (attached). I tried it at the same scale as
used in the paper and got a reasonable results considering the difficulty of vacuum distillation of very small quantities of the methyl ester. I then
tried recovering the free acid as you have done but only managed to obtain a small amount which after recrystallisation gave me only about 0.5g of
glassy fusible crystals ie <10% so you have done well. I will have to try again!
I also tried the same method on aspartic acid in the hope of obtaining cyanoacetic acid but I could not recover any cyanoacetic acid. I didn't try the
ester route. My theory was that cyanoacetic acid is too soluble in water.
I've attached Hiegel's paper for comparison. Note that they have got the name wrong, their glutaric acid is obviously glutamic acid.
|
Yeah i also noticed water was a huge issue in a separate small scale test, atleast with the smaller amino acids. Thats why i used a relatively small
amount of water and also saturated with NaCl. An aquaintance tried with phenylalanine, using a solution of sodium DCCA and solution of sodium
phenylalanine, no issue recovering the product with solvent even without saturating with NaCl.
|
|
|