Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
nitrosobenzene
I would like to prepare nitrosobenzene:
nitrobenzene + Zn + NH4Cl -> phenylhydroxylamine
phenylhydroxylamine + Na2Cr2O7 + H2SO4 -> nitrosobenzene
http://www.orgsyn.org/demo.aspx?prep=CV3P0668
this synthesis requires efficient cooling so I will postpone it to winter time when I can grab some snow outside (much better cooling material than
crushed ice)
this compound is not stable so I will quickly use it for other syntheses:
[0]
Nitroso Diels Alder reaction with dienes (I will use sorbic acid, alternatively dicyclopentadiene which above 150 °C undergoes a retro-Diels–Alder
reaction to cyclopentadiene), I found enough info here, they used cyclohexa-1,3-diene and similar derivates:
https://www.mdpi.com/1420-3049/25/3/563/htm
https://www.mdpi.com/1420-3049/25/3/563/pdf
https://www.mdpi.com/molecules/molecules-25-00563/article_de...
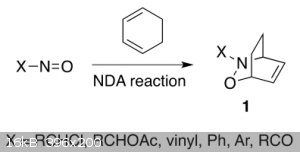
[1]
Condensation with active methylene group (malonic acid ester, benzycyanide) - I did not find any useful info other than this abstract:
https://doi.org/10.1039/B503212C
I was unable to access the full article.
Did anyone perform such a condensation? Do you have any idea of the products of these condensations? Reaction conditions (solvent, catalyst etc)? I
have dimethylmalonate, diethylmalonate, benzylcyanide in stock. Maybe there is no useful product of the condensation? Only guessing as I did not find
anything although I tried to search for a long time. I found only something else where they condensed nitro group (not nitroso), but used sulfur as a
reducing agent and the sulfur seemed to kick off also the CN group (maybe via production of -SCN ?), catalyzed by Fe3+
https://sci-hub.st/10.1002/ejoc.201701607
But the above article is something else than the condensation of nitrosobenzene with benzylcyanide.
Does anyone have any helpful info, any helpful idea? Producing nitrosobenzene itself as a last step could be only a curiosity, it is unstable. I would
like to synthesize something else from it before it decomposes. It is still plenty of time untill winter comes here and brings the snow 
edit: with malonic acid esters this article seems to be useful
https://www2.chemistry.msu.edu/faculty/wulff/myweb26/Literat...
I have also a lot of aldehydes upto C8, there are some reactions in the above article
I have also few kg of L-proline which is able to enantioselectively catalyze condensations
then also various ketones to condense with nitrosobenzene mentioned in the article 
here article about nitrosobenzene + diethylmalonate:
https://www.journal.csj.jp/doi/pdf/10.1246/bcsj.36.870
but still nothing found about nitrosobenzene + benzylcyanide 
[2]
I also found that nitrosobenzene+triphenylphosphine (1:1) could be used for Mitsunobu Esterification Reaction, but I do not expect to perform such
esterifications:
https://sci-hub.st/10.1021/acsomega.8b03551
[Edited on 27-7-2021 by Fery]
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
For the condensation reaction, you seem to be talking about the ehrlich-sachs reaction. I've never done it before, but I've heard of it. The solvent
is methanol and the base catalyst is generally potassium carbonate. Attached is a pdf for a prep.
Attachment: ehrlich-sachs.pdf (172kB)
This file has been downloaded 247 times
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Triflic Acid - thank you very much, that's it !!! 
|
|
|