aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
Ring closing mechanism
I need a little help with a reaction I wanted to do, however, I am worried that I am grossly misunderstanding the reaction I had read about.
Here is what I am thinking
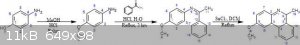
The first two reactions I have reliable procedures for, but the third is going off a 1930s and a 1950s paper talking about ring closure using
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and SnCl2 or SnCl4. They had it cooled to -78C and added the components, then allowing to mix and slow
come to RT. It's been about a month or so since I read the papers and I cannot find them at the moment, I will try to look for them.
Although I do not put it expressly in the reaction above, I read in one of the two papers that an oxidant is added to the reaction or something like
AIBN (Azobisisobutyronitrile).
I am not 100% sure what is happening here, and I am thinking that the Sn is there to coordinate the pi electrons and force it to be planar. This then
can allow a radical oxidative ring closure by way of the hydrogens nearest the imine. The image below from Wikipedia is my basic idea.
I am so far assuming that nitrogen will not pose a problem. I am also assuming that the formation of the ring will break the aromaticity.
 Image source Image source
I do want to do this reaction if possible
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
Lionel Spanner
Hazard to Others
  
Posts: 163
Registered: 14-12-2021
Location: near Barnsley, UK
Member Is Offline
|
|
It looks to me like a variation on a Nazarov cyclisation, with tin coordinating to oxygen and acting as a Lewis acid - not necessarily a radical
reaction.
I suspect the DDQ used in the paper is used to remove the hydrogens at positions 3 and 4, and thereby restore aromaticity, as it's a typical reagent
used for this purpose.
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
Mechanism Idea
Following what Lionel Spanner said,
Here is my go at a possible mechanism for this reaction.
My worry here is that they will not be planar and will not undergo a cyclization.
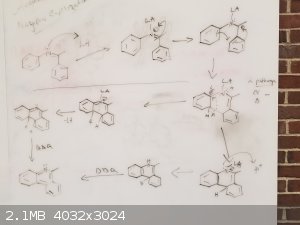
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
Lionel Spanner
Hazard to Others
  
Posts: 163
Registered: 14-12-2021
Location: near Barnsley, UK
Member Is Offline
|
|
It looks pretty plausible, though the first two steps are more likely to occur as a single concerted step. And there's enough rotational freedom in
the molecule for it to form a planar aromatic transition state during that step, which would help a lot in driving the reaction forward.
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
aab18011:
Could you please provide the references to your first two proposed reactions. Both of these steps look very suspect to me, especially the first one.
AvB
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
Quote: Originally posted by AvBaeyer  | aab18011:
Could you please provide the references to your first two proposed reactions. Both of these steps look very suspect to me, especially the first one.
AvB |
Ignore them, this is a file I produced like 4 years ago before I had even taken orgo. I completed the first reaction using 1-bromobutane and
paminophenol to get 4-butoxyaniline. This should be sufficient for the protection. The second reaction on that image was taken from an old research
gate argument about alternative ways to forming schiff bases. My current standpoint on that is that I will use toluene as a solvent and reflux the
components in the toluene. I was going to remake this image to avoid confusion but I got lazy. So forgive me for the odd reactions.
Ps- I have successfully finished Orgo I and II as well as finished the lab portion. Just finished Inorganic and on my way to P-Chem.
[Edited on 12-17-2021 by aab18011]
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
My new notes for the ring closing reaction
Okay, I have been doing some literature research and found some interesting documents on the cyclization of stilbenes and stilbene analogues. The
papers talk about photochemical reactions, but I have found some interesting similarities between the reaction I found in that picture I posted above
and these newer papers.
So heres the gist of it:
The stilbenes can be cyclized using UV light by breaking a double bond into two radicals resulting in a cyclization. The difference though is that I
have an imine analgoue of this. My imine/ket-imine, as discussed in this paper, will not just undergo a cyclization like a standard stilbene. However,
the paper shows that both azo and imine analogues will cyclize if in 98% H2SO4 under UV conditions. The reasoning provided is that there is a
protonation of the imine/azo groups allowing for the double bonds to shift and cyclize.
Now here is my idea: I am thinking that because H+ is a lewis acid, I can do the same reaction by using a stronger lewis acid acid and either
refluxing or using mild UV light to start the reaction. They do include an oxidant, so Id use something like DDQ or maybe a simpler one. They report
38-46% yields.
Here is the reference I am using (I still cannot find the older one I found that I referenced in my reaction schema above):
Mallory, F. B., & Mallory, C. W. (1984). Photocyclization of Stilbenes and Related Molecules. Organic Reactions, 1-456.
DOI: 10.1002/0471264180.or030.01
Note the images attached are of pages 3-10 and page 85. The entire paper is 456 pages and most has nothing to do with my reaction idea.

 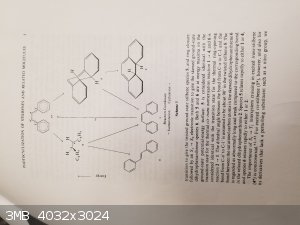
 
 
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
Okay so I have a general theory and Id like as much criticism and discussion as possible:
Stilbene derivatives such as diarylimines can be cyclized by drawing electrons away from the double bond in the imine. From the papers I have read, no
matter if photocyclization or chemical cyclization, they tend to use some lewis acid to attack the nitrogen lone pair. This then causes the carbon
directly attached to take on a positive charge. This then leads to some rearrangement to make this work.
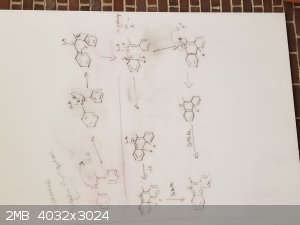 ] ]
My idea here is that we can have any of these bicyclic systems form tricyclic and aromatic systems by utilizing a strong lewis acid and a proper
solvent.
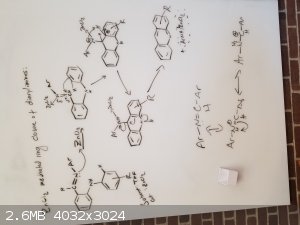
My only issue now seems to be why the original paper calls for a -78C reaction temp that they slowly work up to RT after they add the DDQ
oxidant. My only guess here is that if it was heated up, the cationic carbon would be stabilized by the thermal energy and would not need to
cyclize, possibly because the thermal energy is enough to snap the bond again and reform the carbocation. So therefore, by cooling at -78C, we trap
the tricyclic system and the DDQ quickly aromatizes the rings preventing it from reverting back to where it started. Then after the reaction is
complete and the temperature is brought back to normal, the reaction can be worked up and isolation can take place.
All in all, my idea for the reaction is:
-Prepare Acetone and Dry ice bath
-dissolve the stilbene derivative in THF or Toluene
-also dissolve the DDQ in the reaction mixture
-allow stirring of reaction mixture for like 15 minutes and then slowly bring to RT.
[Edited on 12-18-2021 by aab18011]
Okay so I have been reading furiously and have come across many talks about Sn1/E1 for carbocations. I totally forgot some of the stuff we talked
about in class and am now remembering the importance of heat in the determination whether it is Sn1 or E1. So I am assuming that this also has its
hold on the reaction I am planning. I am assuming that using cold temperatures forces the reaction to find a new path other than elimination. Now
there is no actual elimination because we are using a lewis acid, but the point still stands that lower temperatures favor rearrangement.
[Edited on 12-19-2021 by aab18011]
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
This paper looks interesting
https://pubs.acs.org/doi/abs/10.1021/ol202750u
This paper describes a very similar concept to what I am supposing. Its a cyclization of an alkyne and an imine via a umpolung formed using a
Brønsted base. It seems similar to me because I am suggesting using an umpolung using a lewis acid instead. I guess you could consider the Brønsted
base a lewis acid based off of the mechanism they are suggesting for the paper.
https://doi.org/10.1002/chem.201800219
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
Update:
My professor said that this is 100% possible. He gave me a hint that UV light is not neccessary, but will increase the speed of the reaction. He said
I do not need strong UV light, rather just enough to trap the Z isomer.
He also stated that by adding the oxidant in the reaction mixture before starting, one could effectively force it to completion without UV light. He
suggested testing different UV light wattages as well as testing the addition or absence of the oxidizer, and to test different lewis acids. If anyone
has any suggestions for decently cheap/available lewis acids, it would be of great help. My current thought is SnCl2, SnCl4, AlCl3, H+, and some
chromium lewis acids, probably Cr(6+).
I am currently saving up materials and money, so I will add my reaction notes, procedure, and walkthrough here.
Also if an Admin could guide me in writing a decent writeup here so that it is easy to read and understand for all users of SM, that would be greatly
appreciated. I tend to be squirly with my writing, and I jump around from idea to idea too quickly.
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|