Bender84
Harmless

Posts: 31
Registered: 24-3-2016
Member Is Offline
Mood: No Mood
|
|
Help with FT-IR spectra
Hello World,
Recently I got the opportunity to work with FT-IR, so I'm quite new to this topic. Nonetheless I try to interpret as much as possible by my own and
not to rely solely on the automatic matching software. Usually I'm quite comfortable with the samples I'm receiving to check, but this time I'm really
stuck  (see the attachment). (see the attachment).
Does anyone have an idea what substance this actually may be? Or at least in what group of compounds should I search to try to identify this sample?
I'm quite sure that this is some benzoate but I can't figure out the peak at 3467 cm^-1. I don't know if it's a N-H stretch or water. The sample is a
solid, transparent crystal. Colourless to slightly yellow. It melts at approx. 140*C.
Cheers.

|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Online
Mood: Volatile
|
|
That peak at 3467 would be NH. OH peak is usually broad, round.
From what your sample came from? You should have some basic idea what can be in your sample before analysis.
|
|
|
Bender84
Harmless

Posts: 31
Registered: 24-3-2016
Member Is Offline
Mood: No Mood
|
|
I was told it is some TEMPO derivative. I GUESS it may have something in common with 4-hydroxy-TEMPO benzoate, but the peak at 3467 cm-1 doesn't fit.
I know that OH usually gives a broad band at 3000-3500 cm-1, but a strong sharp peak at 3550-3450 cm-1 is also expected for dimers.
|
|
|
Lionel Spanner
Hazard to Others
  
Posts: 163
Registered: 14-12-2021
Location: near Barnsley, UK
Member Is Offline
|
|
+1 for 3467/cm being an N-H amine stretch. Water would appear as a huge hump obscuring all the other peaks in the range.
The big peak at 1695/cm is a carbonyl stretch.
The large amount of peaks at lower frequencies suggest there's an aromatic system of some kind.
Beyond that you'd need more data to identify it positively - reactivity, NMR, MS, that sort of thing.
|
|
|
Bmoore55
Hazard to Self
 
Posts: 85
Registered: 23-7-2018
Location: Texas
Member Is Offline
|
|
If you can send me the data file I could search my database and see if I could find a match. It looks like you have a great spectra and it shouldn't
be too difficult to search.
|
|
|
Monoamine
Hazard to Others
  
Posts: 160
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
There's a peak near 1700 cm-1 this is a very strong hint that your comopound contains a carbonyl (an oxygen double bonded to a carbon). Also there is
no broad stretching in the 3000-3500 region so you probably don't have any OH or carboxylic acids in your molecule.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Have you run it through AIST. that can help you get a rough match to another spectrum
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Bender84
Harmless

Posts: 31
Registered: 24-3-2016
Member Is Offline
Mood: No Mood
|
|
Hello Everyone,
Thanks for the answers!
I'm aware how the OH peak should look like, but I know that it is not always the case. Sometimes, although not to common, the OH peak is sharp and
looks similar to N-H stretch band. As I wrote I would expect the N-H stretch to be at a lower wavelength.
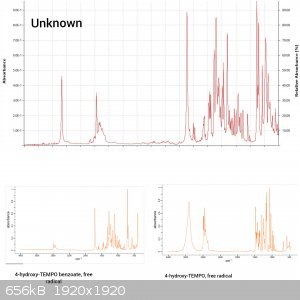
Compare the spectra of 4-hydroxy-TEMP, free radical and its derivative: 4-hydroxy-TEMPO benzoate, free radical. Both have a piperidine ring in their
structure but only 4-hydroxy-TEMPO has a (relative) sharp peak at approx. 3500 cm-1. Therefore I concluded that the peak I'm interested in and present
in the spectra I posted, is due to OH group.
I obtained some "raw" crystals. They were contaminated with some petroleum products/ oil and looked yellow (from the smell I'm sure that in the liquid
covering the crystals definitely some amine was present). I washed the crystals with IPA few times and then few times with demi water. The crystals
were white after washing and looked like sugar. They did not dissolve in IPA or water. They do dissolve in acetone (not to easily, though) and THF
(v.easy).
I dried the product for 24hrs at 100 degC in a lab oven. The crystals were transparent and had a slight yellow tint. I checked the melting point and
it started to melt at 134.9*C. Crystals were fully melted at 147.7*C. I put some washed product in the test tube (1) and melted them few times. The
liquid was orange (2) and after the crystals froze once again they were orange as well (3-7; each time the liquid became darker). I checked the
spectra of the orange crystals and it wasn't to different from the white crystals. So I melted them once again and finally brought to a boil. When the
melt coolled down it was a very dark red and very viscous liquid. I checked the spectra of this substance and there was no noticeable peak at ~3500
cm-1. The rest of the spectrum looked more or less the same. The best match was 4-hydroxy-TEMPO benzoate free radical, although I'm quite sure it is
not the case or at least not the whole story.
I tried to recrystallize the substance in acetone in THF and I got a light orange crystals. Few minutes after the dissolution the solvents turned
pinkish/ orange-ish and took ages to evaporate (probably because I did it in a test tube, because I wanted to obtain larger crystals).
@Traflic Acid
Yes, I did. Thanks to your advice  Unfortunately with no results (I set the
allowance to +/- 30). Unfortunately with no results (I set the
allowance to +/- 30).
@Bmoore55
Thanks! I'll send you spectra (.spa) file.
Best!
Edit: I forgot to mention that the vivid orange crystals on a single picture is 4-hydroxy-TEMPO benzoate free radical (,for visual comparison).

[Edited on 17-12-2021 by Bender84]
[Edited on 17-12-2021 by Bender84]
|
|
|