| Pages:
1
2
3
4
..
12 |
Laboratory of Liptakov
International Hazard
    
Posts: 1334
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
S.C. Wack:
Links are relevants. Only metals (Zn, Ni, Mn) are different. Is it almost CHP. Thus perchlorate metal complex + hexamine + Ammonium perchlorate. Thus
TACP 88% // [Cu(NH3)4](ClO4)2 // + hexamine 6% + AP 6%. It is popular CHP. Thaks for links. Cu(OH)2 + AP + Hexamine under ammonia water sure create
CHP. Is necessary only set exact ratios, temperature, concentrations and time for boiling, I estimate. For all compounds. Easily is use dry TACP, dry
hexamine , dry AP. And mixed under a few drops of NH4OH aq. Heated on 80C and dry. This is classic prepare CHP. Same method with Ni in molecule, not
works, no holes. Tested repeatedly. Zn comlex was not tested.
B(a)P: Thanks for the acknowledgment, I appreciate it ...
[Edited on 14-1-2022 by Laboratory of Liptakov]
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
What the first article says is that when they tried to make CHP by the method that worked for the metals of the second article, they obtained light
blue crystals of hexamine perchlorate instead. (to which a mp of 158C was given long ago FWIW) They say Cu perchlorate hydrolyzed under their
conditions, and that perchloric acid and hexamine gave them AP and HCHO. Which would explain pungent gas, no smell of ammonia, and acid reaction, and
why I wonder about the properties of AP+hexamine and hexamine perchlorate.
(PS mixing perchlorate and alcohol is also something to wonder about)
(PPS I note that DE183355 simply adds 10% CuCl to e.g. K perchlorate/naphthalene to produce a much more sensitive explosive which detonates with
ordinary fuse)
[Edited on 15-1-2022 by S.C. Wack]
|
|
|
yobbo II
National Hazard
   
Posts: 709
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Cannot Copper Perchlorate be prepared using (say) Calcium Perchlorate and Copper Sulphate.
The Calcium Sulphate will PPT leaving Cu Perk.
Ca Perk. can by had my electrolysis.
Barium Perchlorate, Lead Perchlorate and others could be used.
Yob
|
|
|
MineMan
National Hazard
   
Posts: 996
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by yobbo II  |
Cannot Copper Perchlorate be prepared using (say) Calcium Perchlorate and Copper Sulphate.
The Calcium Sulphate will PPT leaving Cu Perk.
Ca Perk. can by had my electrolysis.
Barium Perchlorate, Lead Perchlorate and others could be used.
Yob |
How does this work? How can some perchlorate be made with displacement in water solution but others need perchloric acid?
|
|
|
B(a)P
International Hazard
    
Posts: 1110
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Same as any displacement reaction. If one of the products is insoluble or close to, them it precipitates out leaving the desired product in solution.
Barium perchlorate and copper sulfate would also work.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1334
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
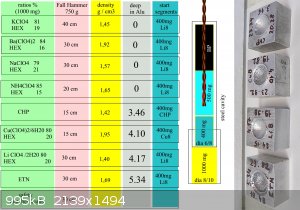
Cu8 and Li8
Due to the discovery of another substance based on perchlorate and hexamine, I enclose a table. I renamed LL8 on Cu8 . The new primary-secondary
substance is referred to as Li8 as a communication abbreviation. The preparation of Li8 is the same as for Cu8. Lithium perchlorate with hexamine has
been shown to give excellent results. And it is even non hygroscopic in laboratory conditions, which is quite surprising. Same as Ba, K, Cu, NH4.
Sodium perchlorate is extremely hygroscopic when mixed with hexamine. The disadvantage of Li8 is can be the availability or price of LiClO4 . 0 - 3
H2O in factory quality.
Interestingly, the preparation of Li8 is free of pungent gas during the evaporation of water and bound water. (Unlike Cu8 where is prepapration
unpleasant) In addition, at Li8 the stainless steel surface can be heated up to 150 Celsius during the final evaporation. Without change of color or
final properties. Absolutely dry Li8 burns with difficulty, purple but it always ignites in the cavity from the wire. Post-explosive gases are
odorless at Li8. Unlike Cu8. Conclusion: Li8 is comfortable primary-secondary substance with 2x less impact sensitivity than CHP and Cu8. For Li8 was
used factory quality LiClO4 .2H2O without acidity in crytstalls. In table are next tested perchlorate mixtures, which are unusable as primary -
secondary compounds. Lithium perchlorate can be manufactured by reaction of sodium perchlorate (relatively easy prepare) with lithium chloride which
can be cheap and available.
Thus, the preparation of LiClO4 may ultimately be easier than the preparation of Cu (ClO4) 2, or the preparation of NH4ClO4 and the subsequent
production of TACP and the subsequent production of CHP. In addition, the final Li8 product is less sensitive to stimuli during preparation and
handling and its detonation pressure is the highest of these perchlorate mixtures.
[Edited on 18-1-2022 by Laboratory of Liptakov]
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1334
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
Li8
Burning Li8 on alu foil

Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
MineMan
National Hazard
   
Posts: 996
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Fantastic work! Li perchlorate has always had a special place in my heart! It’s very special.
|
|
|
MineMan
National Hazard
   
Posts: 996
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
LL. Lithium perchlorate is 2.4g/cc… why is the density so low, at only 1.4g/cc!? This is an anger to the energetic gods!
The oxygen balance of Li perchlorate should allow more hexamine. The perfect mix for AN is 10/90… with AN being +20, the lithium perchlorate being
+64. I imagine the ratio is closer to 25-30 hex. I imagine the chlathrate should be at 1.9-2.0 density pressed. Can you please repeat with 27
hexamine and remeasure the density?
Edit: I did rough calculation, if hexamine is an OB of -205 then your other mixtures are oxygen diffiencet. Therefore, to keep in same tune hexamine
should be no less then 25 percent. Therefore I expect the depth to be 4.5-4.7 if pressed to same density.
Edit 2: it seems I confused the percent oxygen with the OB, it is only 45. Therefore… maybe 20 hexamine is over fueled. But the copper perchlorate
cannot have a better OB?
This is amazing work! I know we are power junkies… Urazine or aminoguanidine should provide even better results, with higher density.
[Edited on 19-1-2022 by MineMan]
[Edited on 19-1-2022 by MineMan]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1334
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
Li8
LiClO4 without water has OB + 60.15 available values. Hexamine - 205.41. Utility counter show ratio 77 : 23 with OB - 0,929. But was used
LiClO4 x 2H2O. Therefore was added 3%. Thus ratio 80:20. Also line of powder in alu foil V shape long 7 cm show best speed at ratio 80:20. Was
pressed a new cylinder. Height 1,240 cm in diameter 8,00 mm. Pressed in 4 steps in vise on maximal density while maintaining the diameter of the
cavity 8 mm. Density of 1000 mg in this cavity
should by 1.605 g/ cc. It can't be more in amateur conditions. 4x twisting the rod from cavity is Russian roulette.
Of course, the ratios for Li8 and Cu8 can be calculated more accurately during further research. Urazine a aminoguanidine is research of Microtek.
I have only 2 hands.


[Edited on 19-1-2022 by Laboratory of Liptakov]
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
Microtek
National Hazard
   
Posts: 827
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
| Quote: |
I have only 2 hands |
I know exactly what you mean. The number of things that needs to be investigated can sometimes be overwhelming.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1334
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
Exactly. An ordinary one table with results like here above will take up all free time for the whole week. For provide results free. If such a job
were done by a professional laboratory, results in table would be cost thousands of dollars.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
MineMan
National Hazard
   
Posts: 996
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Laboratory of Liptakov  | | Exactly. An ordinary one table with results like here above will take up all free time for the whole week. For provide results free. If such a job
were done by a professional laboratory, results in table would be cost thousands of dollars. |
Probably ten thousand. But don’t worry LL, when I hit the big bucks you can work in my lab and do this professional  all the chemicals you want, including ethanol and DMT! all the chemicals you want, including ethanol and DMT!
Shouldn’t the density be higher still, for example 2.1g/cc? Anyway to drive the water from the Li perchlorate? In fact it’s not a dry mix, it’s
a ligand , water should be removed? Tho I have no knowledge. Burning spending unconfined does not relate to Detonation performance we know.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1334
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
Li8 Cu8
Burning rate Li8 on air is for firsts attempts important. Li8 is quite difficult to ignite. It goes out without support. If Li8 is to pass the test
as a monomaterial, thus primary-secondary material, it must be possible to ignite it in the cavity reliable. Without other ingredients. Combination
with CHP or Cu8 or ETN or Alu or BP powder may be the subject of further research. But at ratio 80:20 (at density 1,4 +) is presure enough high for
starting any secondary EM. Which is basic purpose.
Higher density for Li8 require special pressing device, tools, equipment. For example, aminoguanidine is not simple for preparation. So I leave such
substances to others. Bay the way, Anyone can work on improvements of Li8/Cu8. Not just me.
Li8 and Cu8 are basic research products. Similarly a like CHP.
Applications can be mixtures with similar ratios. However, they rely on successful substances from the basic research.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
MineMan
National Hazard
   
Posts: 996
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
I lost my post.
I want this to out perform ETN.
Why is the density so low? Is this ligand much less dense than a dry mix.
I ask, can it for eutetic when heated, just like AP and Hex? Euteitc or melt will be most density. There is a good chance, if it works with AP it
could work with this, especially if water is present. I want the holy 2.0 of density.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1334
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
Is it weird, the low density. 2,42 80% + 1,33 20% should by 2,20g / cm3...
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
MineMan
National Hazard
   
Posts: 996
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
You have to use harmonic density calculations instead. Nonetheless, this is close enough. At 2.2 density, should easily outperform ETN if it’s close
at 1.6.
|
|
|
C6(NO2)5CH2CH(CH3)N(NO2)2
Harmless

Posts: 43
Registered: 4-4-2018
Member Is Offline
Mood: No Mood
|
|
I wonder is this a clathrate, complex, double salt, mixture or if it actually formed a new compound? the part about irritating gases coming out makes
me think there may be a new organochlorine compound or something.
It would be great if a bigger commercial or government lab could give it a try and try to characterize the structure of it.
Put that in your pipe and smoke it!
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1334
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
The amateur assumption is that Cu8 is mixture of copper-hexamine - diperchlorate + copper perchlorate in one crystal + small amount copper hydroxide,
about 1%.
And some similar is Li8. Important is, that is works very well both. Because result of attempt is 10x more important than theory.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1334
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
Li8 and Cu8
For sure was tested mixture Li8 + Cu8 50:50. Brisance is bigger than CHP, but weaker than at others on picture. Pretty surprisingly is, that Li8 is
difficult fired as free heap. During a 2 second goes out after ignition. But detonation pressure of Li8 is the highest of all examined mixtures. I
don't know of any other primary mixture (or primary-secondary) that will go out on its own if you ignite it. For reliable DDT of course Li8 require
solid metal cavity. From unknown reason is surface of crater from Li8 pretty smooth. Against all others. And after test was this crater as only one
clear, without black residuum. Explosive fumes were also odorless only at Li8. But that has been mentioned before.
List in brizance order:
ETN ...........5,34 mm
Li8..............4,17 mm
Cu8............4,10 mm
Li8/Cu8......3,67 mm
CHP...........3,46 mm

Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
BTW US3066139 (which has little data) bears a slight resemblance to this, with Al nitrate and K chlorate as the preferred additives.
[Edited on 23-1-2022 by S.C. Wack]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1334
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
There are hundreds of different mixtures with hexamine. Hundreds of old patents on similar theme. But accurate test is crucial. And importantly, don't
be afraid to mix it. And to press it. Only then will the results come....
Attachment: Kruszynski2015_Article_OnTheCoordinationBehaviourOfTh.pdf (971kB)
This file has been downloaded 397 times
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
B(a)P
International Hazard
    
Posts: 1110
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
I hope this is the correct place to post this. I am certainly not trying to hijack this amazing thread.
Inspired by LL I decided to experiment with nickel perchlorate and hexamine.
After some experimentation I am not sure this mixture will be of use. I used the same method as described by LL in the original post on this thread to
combine the reagents. The issue is that the mixture is so hygroscopic it is impossible to get dry. On heating the mixture will become a sticky toffee
consistency. I have tried heating very small amounts to complete dryness, but it decomposes before it is dry. I am currently trying to dry a sample of
the mixture in a desiccant box. Unfortunately I do not have the capability to dry under vacuum, but I might try and rig something up if the desiccant
box does not work. Any suggestions to help dry this mixture and allow some testing would be most welcome.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1334
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
Li8 - Lithex
There is no mention of energy properties in the attached Kruszynski link. This link was found back only after verifying the energy properties of Li8.
It can be used to form said ligand.
Similar method of preparation: 80% LiClO4 dihydrate x 2 H2O + 20% hexamine. Dissolve 4 g of LiP + 1 g of hexamine in 5.2 g of dH2O. Heat to boiling
point and cool in an ice bath. The solution almost "solidifies". The resulting slurry is filtered off. Fine needle crystals remain on the filter.
Color and consistency like snow. Vacuum suction is a condition. The crystals thus formed are transferred to a stainless steel pan. The temperature is
adjusted to 80 DEG C. At this temperature, the crystals soon dissolve, a liquid is formed. The liquid is a dihydrate-ligand. When the temperature is
raised to 100 C, the dihydrate dries. And it becomes a monohydrate - a ligand. When the temperature is raised to 120 ° C, the monohydrate dissolves
again. When the temperature is raised to 150 C, the monohydrate dries. The material requires constant friction with a steel plate in all steps. The
preparation of Li8 is not for the faint of heart. It requires strong nerves. After drying at 150 DEG C., the temperature is reduced to 80 DEG C. And
at this 80 C is filled material into the cavity. Li8 anhydride was shown to be hygroscopic. Arised monohydrate overnight, at room conditions.
Monohydrate looks a like dry powder. But at increase temperature on 120 C created solution. LiClO4 can be trihydrate, dihydrate, monohydrate,
anhydride. Same behavior are for Li8. Next method for preparation Li8 is create anhydride of LiClO4. And after mixing 77 % + 23% of hexamine. Add dH20
to full dissolve at 100 C. And increase temperature on 150 C to total dry powder. I recommend working with an amount of about 1 gram.
Gloves, shield, ear plugs. First described method with filtering provide better results for finally brisance.....
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1334
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
Lithex
B(a)P...interesting. Important attempt. Now we know, that Nickel not works. Every different metal - perchlorate has different behavior in mixture
with hexamine. It seeems, that stabile ligand can create
only Cu, Li, Na perchlorates + hexamine. In Kruzsynski (link) was tested a lot exotic metals. But only Lithium and Sodium created ligand.
Copper was nort examined there.
Preparation must be easy. Without desiccators and the like. The Li8 turned out to withstand really rough handling. And a long heating, for example, 2
hours at 120 Celsius. Then it has even the best features. And is burning easy as heap. CHP would not be able to withstand such treatment. It would
either explode or disintegrate.
I consider Lithex (Li8) to be a luxurious primary-secondary substance. And despite the fact that he is hygroscopic. Accurate measurements showed that
3.01 g of LiClO4 x 2 H2O contained 2.47 g of LiClO4 anhydride. 3g therefore contain 22% water.
OB for LiP is + 60.15. The OB for hexamine is -205.41. From this follows the ratio
77 parts dry LiP + 23 parts dry hexamine. Where the resulting OB = -0.929.
Thus, the exact ratio of LiP dihydrate should be 2.97g + 0.69g dry hexamine. Beware, hexamine is also moist often. It must be dried before weighing.
Because LiP is up to trihydrate, we never know exactly how many grams to use for the exact ratio. For this reason, it is much easier and safer to use
LiP anhydride and dried hexamine. In ratio 77:23. As already indicated, at least 2 crystalline barriers must be overcome when drying LiP. At 80 and
120 C. The melting point of completely dry LiP is 236 C. Boiling point - decomposition 400 C. For this reason, there is no need to worry about
decomposition during drying to the anhydride. Caution, do not use Teflon for drying. LiP immediately reacts with Teflon at 80 ° C and begins to
blacken. Even the stainless steel surface is not perfect. When LiP is melted, there is a slight browning of pure factory LiP. The same effect is
observed when the Li8 mixture is dried. After drying, the mixture is slightly gray. However, its energy properties will not affect it for the worse.
Humidity affects Li8 performance quite fundamentally. Therefore, all tools must be kept warm around 40 C during handling and filling. And work
quickly.
If all this is adhered to, Li8 provides surprising results.
The preparation is odorless and even the fumes after the test are odorless. Repeatedly measurement confirmed low sensitivity to impact, ie a 750g
hammer from a height of 30 cm, initiation in 50% of cases. Like ETN. CHP is initiated from 15 cm under the same conditions. Friction sensitivity of
Li8 has not been tested. But since Li8 is rubbed with a flexible steel at 150 C on a fine powder, the sensitivity to friction will not be high.
[Edited on 24-1-2022 by Laboratory of Liptakov]
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
| Pages:
1
2
3
4
..
12 |