| Pages:
1
2 |
DocX
Hazard to Others
  
Posts: 179
Registered: 22-10-2015
Member Is Offline
Mood: No Mood
|
|
Separating OTC Xylene
So the commercially available Xylene where I'm from is mixed with 10-20% ethylbenzene. The boiling point is too close for an easy distillation, but
looking at the melting point it seems like it would be easy to separate them by freezing: ethylbenzene has a melting point of - 95C, while p and
o-xylene has 13 and -24C respectively. M-xylene has -48C though, which is beyond the scope of my freezing technology. Which is next to the shrimps and
icecubes.
Is this as easy as it looks, or are there difficulties hidden there for my noob eyes?
And is there a better way to purify the xylene mix? This way I should be able to separate the isomers also, right?
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
You can always try sticking it in the freezer and see if anything crystallizes out, but the likelihood of that is slim to none, because the higher
melting isomers will probably remain dissolved in the lower melting ones. There's likely also melting point depression involved where the mixture will
have a lower melting point than any of the constituent isomers. I'm pretty sure that nobody here has had much success purifying mixed xylenes. In
order to affect distillation, you'd need a really, really efficient column, and even if you had that it would likely take days of continuous
distillation to obtain small amounts of the pure isomers. Not really feasible on a home lab scale.
|
|
|
DocX
Hazard to Others
  
Posts: 179
Registered: 22-10-2015
Member Is Offline
Mood: No Mood
|
|
That's more or less what I suspected. But then I saw this lecture by an Indian chemistry professor who mentioned freezing point as a viable way to
separate xylene isomers.
Apparently I can isolate at least the p-xylene by freezing crystallization. From PubChem:
| Quote: |
Low temperature fractional crystallization was the first and for many years the only commercial technique for separating PX /4-xylene/ from mixed
xylenes. ... PX has a much higher freezing point than the other xylene isomers. Thus, upon cooling, a pure solid phase of PX crystallizes first.
Eventually, upon further cooling, a temperature is reached where solid crystals of another isomer also form. This is called the eutectic point. PX
crystals usually form at about -4 °C and the PX-MX /4-xylene-3-xylene/ eutectic is reached at about -68 °C.
|
But the main thing is separation of xylene from ethylbenzene though. So if thats possible by freezing, I'm happy.
Maybe I'll just stick it in the freezer and return with an experimental section  .
First though I might get some dry ice and try to get the m/o portion in a second run. .
First though I might get some dry ice and try to get the m/o portion in a second run.
[Edited on 20222222/1/11 by DocX]
[Edited on 20222222/1/11 by DocX]
[Edited on 20222222/1/11 by DocX]
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
I did some experiments on the purification/separation of xylenes.
For freezing-out p-xylene the xylene mixture should be dissolved in methanol (that is what some literature says). I did an experiment mixing 20 ml of
purified xylene mixture and 30 ml of methanol but nothing precipitates after keeping few weeks at -18C, so, I will try to dilute it more.
I was not aware of ethylbenzene but I had experience with the presence of sulfur compounds. When I used commercial xylene to store Na metal the metal
becomes covered with a yellow gelly mass. So, I did purification by shaking with conc. H2SO4, etc, drying, and fractional distillation from sodium
metal (by mixing procedures from Vogel's handbook and Perrin & Armarego & Perrin). Now it is pure enough to store Na metal under it.
But I noticed (when I did the fractionation) that my sample of commercial xylene contains mostly lower boiling point components, so the o-Xylene part
is minimal - I've got the raising of the temperature only near the end of the distillation.
As a side observation, I found that xylene & methanol mixture is very good for cleaning glassware after mineral oil, so now I keep it for that
purpose. Probably it could be used for cleaning e.g. lenses/telescope mirrors also with better results than OTC xylene (which is traditionally used
for that purpose).
[Edited on 12-1-2022 by teodor]
[Edited on 12-1-2022 by teodor]
|
|
|
DocX
Hazard to Others
  
Posts: 179
Registered: 22-10-2015
Member Is Offline
Mood: No Mood
|
|
[Edited on 20222222/1/12 by DocX]
|
|
|
DocX
Hazard to Others
  
Posts: 179
Registered: 22-10-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by teodor  | | For freezing-out p-xylene the xylene mixture should be dissolved in methanol (that is what some literature says). I did an experiment mixing 20 ml of
purified xylene mixture and 30 ml of methanol but nothing precipitated |
I’m sorry, but WHY should it be diluted with methanol? That ought to lower the freezing point significantly, no wonder nothing crystallises? Have
you tried without it?
[Edited on 20222222/1/12 by DocX]
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Of course, I tried - why do you think I started to use methanol?
I suspect that p-xylene has a lower solubility in methanol than in o/m-xylene, that's why this method is recommended.
Anyway, this is the citation from "Perrin & Armarego & Perrin":
"p-Xylene can readily be separated from its isomers by crystn. from such solvents as methanol, ethanol, isopropanol, acetone, butanone, toluene,
pentane or pentene..."
So, the question which I tried to solve is not whether it is possible but which proportion between xylene and solvent should be used.
[Edited on 12-1-2022 by teodor]
|
|
|
DocX
Hazard to Others
  
Posts: 179
Registered: 22-10-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by teodor  | Of course, I tried - why do you think I started to use methanol?
So, the question which I tried to solve is not whether it is possible but which proportion between xylene and solvent should be used.
[Edited on 12-1-2022 by teodor] |
Ah, ok. Are you using tech grade xylene with ethylbenzene in it? I'm thinking the eb might be reason. Maybe it forms an eutectic mixture with p-xylene
that lowers the melting point drastically. It seems to be an issue even with the pure xylene mixtures:
| Quote: |
Among the three isomers of xylene, p-xylene
has the highest melting temperature at Tm= 13.3 o
C
and, for this reason, can be separated by fractional
crystallization, with a recovery rate of only 50 % of
the p-xylene present in the mixture. This process is
affected negatively by the formation of a eutectic
mixture of xylene components.
|
/Santos et al, Separation of Xylene isomers, Brazilian journal Of Petroleum And Gas, 2011.
Maybe wrapping it in dry ice though? I'll try that as soon as I can get some.
EDIT: and then there's this: https://www.jstage.jst.go.jp/article/jpi1959/16/1/16_1_43/_pdf
I haven't had time yet to check the probable crystallization temp from the graphs in the article for the molar ratios given in OTC xylene, but since
it's -40something in a mixture consisting of mainly m-xylene, probably it would be lower with a mix where p is dominant. But then there's the
ethylbenzene. The eutectic temp with an ethylbenzene mix is given in the quarternary phase diagram (fig nr 5), but I have a hard time reading it.
[Edited on 20222222/1/12 by DocX]
[Edited on 20222222/1/12 by DocX]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
There is a great deal of information out there on the utilisation of mixed isomer xylene but you have to decide what it is you want to do with the
separated isomers. I older times the mixture was nitrate and reduced to the mixed mono amine and then these were separated by a complex series of
processes. If you are interested I try and dig out the references. Other procedures are based on the selective sulphonation or oxidation to remove
only one or two components from the mixture. Complete oxidation to phthalic acid isomers and then fraction crystallisation can be used but this
presupposes that you want said acids.
What does the OP actually want to do? Simple separation is very difficult and probably not worth the bother. I did a bit of work on selective
sulphonation some years ago, its was a lot of work and reagents for a small yield but interesting practice 
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
DocX, I don't quite understand how this eutectic mixture behaves.
I understand that adding a lower freezing point component will lower the freezing point of the mixture. But can it work in the opposite way, when
adding some higher freezing point component will higher the freezing point of the mixture?
As for sulfonation, I also wanted to try this. Just for educative purposes, to compare with sulfonation of toluene and benzene. If we can get
separation of isomers also during this process it would be even more interesting.
|
|
|
Newton2.0
Hazard to Self
 
Posts: 63
Registered: 12-8-2019
Member Is Offline
|
|
Having just discovered that toluene and water form an azeotrope that separates readily in a Dean-Stark apparatus, I wonder if o-, m-, p-xylenes have
unique properties that would allow one to favorably extract a particular isomer with the correct choice of solvent? Then keep repeating the process
and saving the leftovers until you get your desired compounds out of the mix.
Another method I wonder about is by performing certain types of FC alkylations with the idea being that the steric bulk of osme isomers being less
favored and unreacted isomers being likely remnants after completion.
|
|
|
Alucard
Harmless

Posts: 22
Registered: 6-2-2019
Location: European Union
Member Is Offline
|
|
Quote: Originally posted by teodor  |
As for sulfonation, I also wanted to try this. Just for educative purposes, to compare with sulfonation of toluene and benzene. If we can get
separation of isomers also during this process it would be even more interesting. |
You will actually end with a mix of p-xylene and ethylbenzene after done. m-xylene might be sulfonated much easier than other things in xylene mix,
after there is no longer meta isomer present, you can continue with removing ortho isomer by sulfonation process, what is left is actually p-xylene
and ethylbenzene, but still it's not possible to say freeze out p-xylene from this mix by using say domestic fridge with -20..-25 range.
I saw a paper that claims it is possible to separate p-xylene from ethylbenzene by azeotropic distillation with p-dinitrobenzene, it sounds
interesting, but I have no access for this paper and saw no details about this process.
I think it must a better option to simply buy p-xylene, because separating xylene mix requires a lot of work, and I'm not sure it contains only three
xylene isomers and ethylbenzene, there might be some gasoline present along with other products. Who knows how much of crap is present in paint
thinner grade xylene mix...
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Newton2.0, Dean-Stark works for azeotropic mixtures of compounds that are not miscible with each other and the unwanted component is denser.
Saying that I'd like to point that an azeotropic mixture is formed due to the properties of vapor pressure/temperature curves of 2 or more compounds.
That means that compounds that have almost the same vapor pressure/temperature curves probably will form the same azeotropes.
So, the main question is, how different are vapor pressure/temperature curves for the 4 main compounds of OTC xylene (p-,o-,m-xylene, and
ethylbenzene).
And if they are really different somewhere, just lowering pressure during fractionation will increase the boiling point difference.
As for a practical method, I think sulfonation with separation of o-Xylene (which is most readily sulfonated) and further work with the mixture of
p-,m- & ethylbenzene is the thing to try. Yes, it requires 1 volume of conc. H2SO4 per 2 volumes of OTC xylene (according to the procedure I have)
but even H2SO4 is a more available component for me than dry ice - I have no idea where to get dry ice and how to store it.
Update: I checked vapor pressure/temperature dependency for o-Xylene, p-Xylene, and ethylbenzene by the tables I have (Timmermans, Physico-chemical
constants of pure organic compounds) and I don't see any significant differences between them.
Update 2. See what I found:
Attachment: patterson1924.pdf (229kB)
This file has been downloaded 191 times
And this is the second, complementary, work:
[Edited on 13-1-2022 by teodor]
Attachment: clarke1923.pdf (319kB)
This file has been downloaded 183 times
|
|
|
DocX
Hazard to Others
  
Posts: 179
Registered: 22-10-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by teodor  | DocX, I don't quite understand how this eutectic mixture behaves.
I understand that adding a lower freezing point component will lower the freezing point of the mixture. But can it work in the opposite way, when
adding some higher freezing point component will higher the freezing point of the mixture? |
I wouldn't presume to understand it fully either. But from the information given in the article I referenced, my impression is that the relationship
between the different isomers and ethylbenzene drastically affects the freezing point of one or the other. That is, the eutectic temp.
So as p-xylene crystallizes out, the mixture changes composition and with it the freezing temp.
From the graphs and the text I understand that as the proportion of p-xylene in the mixture increases or decreases, so does the melting point. I can't
however figure out what effect the ethylbenzene has, although I'm sure the information is in there for smarter minds than mine.
And for those wondering what it is I want to do:as I wrote in my second entry, what I really really want is to get rid of the
ethylbenzene. As swiftly and simply as possible. To produce somewhat pure xylene.
For bonus points it would be great to be able to separate p-xylene to make phtalic anhydride from it. But that's not absolutely necessary. Just cool.
[Edited on 20222222/1/13 by DocX]
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
I do some experiments with it, but I already have the impression that OTC xylene which I have has already p-xylene contents removed or lowered
compared to the composition you referred to.
|
|
|
Syn the Sizer
National Hazard
   
Posts: 591
Registered: 12-11-2019
Location: Canada
Member Is Offline
|
|
According to this paper ethylbenzene forms an azeotrope with water and comes over at 33.5C but needs to be under vacuum of 80mbar.
m-Xylene also forms an azeotrope at 94.5C and doesn't require vacuum.
I almost forgot to link the paper, I hate to reference a paper and not link the paper.
https://doi.org/10.1002/14356007.a28_433
On a side not, the water/toluene azeotrope is 79% toluene, m-Xylene azeotrope by the linked paper is is 60% m-xylene would that make m-xylene a better
choice for use in a Dean Stark. Not that I am about to fork out the extra cash for m-xylene when I have toluene OTC, but just a thought.
Though I guess there is more at play such as the aromatics density too and other factors.
Syn'
[Edited on 14-1-2022 by Syn the Sizer]
[Edited on 14-1-2022 by Syn the Sizer]
|
|
|
Newton2.0
Hazard to Self
 
Posts: 63
Registered: 12-8-2019
Member Is Offline
|
|
teodor, thanks for the update, I am quite new to this concept but it is fascinating. This is education that I wouldn't have gotten elsewhere, so I
appreciate you.
I have a mixture of CHCl3 and Toluene and a simple distillation was not enough to separate these two. I know this is off-topic, but I may give this a
try.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Chloroform and toluene? Those are 50 degrees apart, water would only give two azeotropes which are closer together. Or do I misunderstand what you are
saying?
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Syn, that's amazing, but could there be tertiary and more components azeotropes when water is added to the mixture of xylenes?
Also, I have the impression that my OTC xylene is 70-90% m-xylene and 6% paraffins, not counting sulfur compounds which bothered me most, but they are
already separated. I will write when I will have more information.
[Edited on 14-1-2022 by teodor]
|
|
|
Syn the Sizer
National Hazard
   
Posts: 591
Registered: 12-11-2019
Location: Canada
Member Is Offline
|
|
Quote: Originally posted by teodor  | Syn, that's amazing, but could there be tertiary and more components azeotropes when water is added to the mixture of xylenes?
[Edited on 14-1-2022 by teodor] |
Sure the possibility could be that there is a component that could for a ternary azeotrope, however I did not find any info on a ternary azeotrpe
involving water and m-xylene, but doesn't mean it doesn't exist.
My guess is of the most common solvents used, there is none that will form a ternary azeotrope.
Edit:
Little more searching found this paper
https://pubs.acs.org/doi/pdf/10.1021/je990321u
The abstract is saying p- and m- isomers will form a ternary azotrope with water and ethanol
Another side note, maybe this would be an easy way to separate isomers.
Boil mixed xylenes with H2O and ethanol and distill over all of the m- and p- as the azeotrope (I didn't look at the paper itself just the abstract so
unsure of the ternary bp) which would leave the o- isomer in the still pot.
From there run a Fisher esterification to form ethyl hexanoate with a dean stark bp 168C. This would carry over the m- isomer into the dean stark at
92C to as the binary azeotrope leaving the p- isomer and ethyl hexanoate in the still pot.
From there fractional distillation would be best to get the p- isomer bp 138 separated from the ester as the difference in bp is only 30C, sure you
can if careful use simple distillation for this.
Again this is just a thought, more often then not nobody needs separated isomers of xylene when used as a solvent.
Syn'
[Edited on 14-1-2022 by Syn the Sizer]
[Edited on 14-1-2022 by Syn the Sizer]
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
According to the article you referred to in your last post, o-,m-,p- xylenes all form azeotropes with water with almost the same boiling points. So,
the question also is about the data reliability in the references.
OK, let's say we get some azeotrope with water. How do you propose to check we get m-xylene only?
[Edited on 14-1-2022 by teodor]
|
|
|
Syn the Sizer
National Hazard
   
Posts: 591
Registered: 12-11-2019
Location: Canada
Member Is Offline
|
|
Quote: Originally posted by teodor  | According to the article you referred to in your last post, o-,m-,p- xylenes all form azeotropes with water with almost the same boiling points. So,
the question also is about the data reliability in the references.
OK, let's say we get some azeotrope with water. How do you propose to check we get m-xylene only?
[Edited on 14-1-2022 by teodor] |
Hmm, there should be no azeotrope with water for the p- and o- isomers, that is the reason toluene is used for Dean Stark apparatus even though it is
harder to get for some people.
I will look through the paper and see where it says this and then check the references to see what the paper referenced as mentioned I just read the
abstract, and have known for some time that only the m- isomer does form an azeotrope.
One method that could be used to test easily at home is oxidation of the distillate, phthalic acid is slightly soluble in H2O, teraphthalic acid and
isophthalic acid are insoluble.
|
|
|
Syn the Sizer
National Hazard
   
Posts: 591
Registered: 12-11-2019
Location: Canada
Member Is Offline
|
|
To be honest, I would say their data is flawed. Look at any azeotrope table and it will show only m-xylene producing an azeotrope.
Their reference can't be found on z-lib.org or anywhere else free, but available on Amazon for over $400. But I keep checking more tables and none
have a p- or o- isomer forming a binary azeotrope.
This is the book they reference
| Quote: |
(7) Gmehling, J.; Menke, J.; Krafczyk, J.; Fischer, J. Azeotropic Data,
Part II; VCH: Weinheim, 1994.
|
and the table from the paper I linked showing the reference as well.
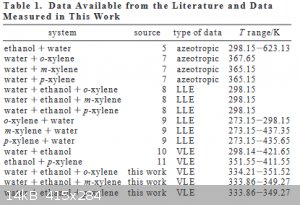
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Yes, I did attempt to find that book too. The tables published by the American Chemical Society (available at z-lib.org) show water azeotrope for
m-xylene only.
But I have no way to know which data is more accurate - ACS or in that book. So, I am a bit suspicious also because I don't understand why compounds
with almost the same vapor pressure/temperature curves selectively form azeotropes with water based on the structure of the molecule only.
Also, according to the paper, all of them forms azeotropes water-ethanol-(p,m,o)-Xylene, which also supports the version that all 3 forms form also
similar azeotropes with water only.
OK, I dig a bit into theory and I see that if that is really the case that only m-xylene forms an azeotrope with water that should mean there is some
unique interaction between m-xylene and water molecules in a liquid phase which is absent for other isomers.
Update: funny, this topic was already discussed here:
http://www.sciencemadness.org/talk/viewthread.php?tid=69528
The user Maroboduus supported the version identical to my own there.
And what is strange. ACS tables have a row "Nonazeotrope" for many compounds. But they have no any "Nonazeotrope" record for p-, o- Xylenes, they just
skip them.
[Edited on 14-1-2022 by teodor]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Syn the Sizer  |
One method that could be used to test easily at home is oxidation of the distillate, phthalic acid is slightly soluble in H2O, teraphthalic acid and
isophthalic acid are insoluble. |
And ethylbenzene will give benzoic acid. It shouldn't be too hard to separate the acids (especially if one of the acids is your end goal).
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
| Pages:
1
2 |