Monoamine
Hazard to Others
  
Posts: 160
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Question about Friedel Crafts acylation and acid chlorides
I'm planning to do my first acylation experiment soon, but I've never done this reaction, so I'm a bit unsure on some of the specifics.
1) I will make the acid chloride using propionic acid and thionyl chloride. I was planning to just reflux the acid in excess SOCl2 for an hour.
I'm a bit worried that I'll accidentally make propionic anhydride, instead of just the acid chloride. Is there a way to prevent the formed acid
chloride from reacting with the the propionic acid and making an anhydride instead?
2) I want to acilate naphthalene, and as it turns out, the site of acilation can be controlled by the solvent that is used. Solvents like
dichloromethane tend to favour substitution at the 1 position, while nitrobenze or nitromethane strongly favour substitution at the 2 position. I want
to test this
The thing is that I don't know for how long to run the reaction for and at what temperature it should be carried out.
Any help would be greatly appreciated! Thank you in advance.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2658
Registered: 26-1-2011
Location: USA - NC
Member Is Online
Mood: No Mood
|
|
If you use excess thionyl chloride, any anhydride would break up to form the chloride. I usually use the thionyl chloride as the solvent, so there is
lots of extra. You can distill or rotovap off the excess to recover it if you wish. But you must remove the excess thionyl chloride before moving
on, either on a rototvap, distillation or high vacuum.
If in doubt, start cold and then slowly warm the reaction, until TLC shows some changes. Then heat if needed and allow the reaction to proceed until
TLC shows no change. That is how most reaction times are decided, by testing for change or loss of SM.
Naphthalene should be easy to see by TLC, along with the ketone formed. Are you using a FC catalyst of some sort?
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Monoamine - alfa as well beta acetonaphthones have nice scents, perhaps propionaphthones too - I did not find info about their scents.
Anyway also propionic anhydride is capable of Friedel-Crafts with anhydrous AlCl3 or FeCl3 catalysts.
See example 3 from the attachment (but there acetic anhydride instead of propionic)
https://patents.google.com/patent/US2487777A/en
Attachment: US2487777A.pdf (281kB)
This file has been downloaded 193 times
|
|
|
Dr.Bob
International Hazard
    
Posts: 2658
Registered: 26-1-2011
Location: USA - NC
Member Is Online
Mood: No Mood
|
|
But for the anhydride, you typically need 2 equivalents, rather than 1 for the chloride. That is the main reason to use the chloride.
|
|
|
Monoamine
Hazard to Others
  
Posts: 160
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Thank you for the tips!
I'm planning to use anhydrous AlCl3 as the catalyst. From what I read, the AlCl3 will form a complex with the formed ketone so I think I'll have to
use at least 1 mol equivalent to the acid chloride to allow the reaction to go to completion.
This should make it more clear:
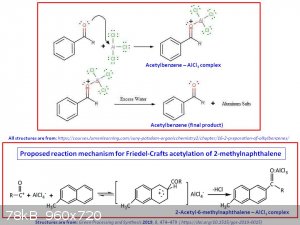
Also, now that I think of it, I guess one way to check that the reaction is done (in both cases) is to see when there is no more HCl evolving from the
reaction.
Here's the reference for the reaction:
Solvent effects on the acylation of naphthalene
[Edited on 3-2-2022 by Monoamine]
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi Monoamine, very interesting link, that solved also my questions. So alpha isomer produced quickly (kinetic reaction) in dichloromethane as its
AlCl3 complex insoluble in dichloromethane which is then final stage of the reaction. But the quickly formed alfa isomer complex with AlCl3 soluble in
nitrobenzene so the quickly formed alfa isomer then slowly proceeds to more energy-stable = beta isomer (thermodynamic reaction).
I bought quite cheap AlCl3, acetyl chloride, SOCl2, nitrobenzene from this supplier, I see you are in EU so no customs controls as the seller is from
EU too:
https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
I hope propionyl products from naphthalene would have also nice scents, as acylation products are orange like scents. Also propiophenone has somewhat
nicer scent than acetophenone although acetophenone is also nice. But that is only my speculative guessing, it should be tried in real experiment.
Very interesting, keep up your good work.
|
|
|
Monoamine
Hazard to Others
  
Posts: 160
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Hi Fery, thank you for the link for the reagent supplier! My mouth is watering seeing all the wonderful reagents and structures they have.
I've only had the chance to smell a limited number of formylated aromatic compounds, but the ones I have, including indole, all seem to have a kind of
marzipan smell. Wonder if naphthalene will be the same. Then again I'm aiming for a propyonyl, and naphthalene itself has a very strong smell. Guess
we'll find out...
|
|
|
Monoamine
Hazard to Others
  
Posts: 160
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Analysis
So I finally tried this experiment and got a decent looking brown oil that had a smell of orange peels with a hint of benzaldehyde. IR scan showed a
clear carbonyl absorbance peak at 1700cm-1 plus some aromatic stuff so the acylation itself worked.
I realized later that I should have done the reaction at around -10C instead of the 60C or so that I ran it at, so I expected that I wouldn't just
have one isomer. I did some TLC to get some idea of the composition. Since I don't have much experience with TLC I don't know 100% what to make of it.

A mixture of hexane and ethyl acetate was used as the mobile phase.


The plates are viewed at two different UV light wavelengths.
Considering that there are both orange and blue spots, I would guess that each colour is one of the isomers. I'm guessing the blue one is less polar
and very soluble in ethyl acetate since it travels further and diffuses through the solvent the more ethyl acetate is in the mobile phase. But I can't
tell if the naphthalene-1-propanone or the naphthalene-2-propanone would be the less polar isomer.
It's not clear to me though which solvent system gives the best separation. I'm guessing 80% hexane and 20% ethyl acetate?
Would anybody here know which solvent system to use for attempting to run a column? Curious to hear what other folks think.
Also, why would one of the isomers fluoresce blue and the other orange?
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi Monoamine, very nice!!! So did you perform the synthesis using dichloromethane as a solvent? If some naphthalene stayed unreacted, it would be the
least polar compound. In DCM at low temp the complex of AlCl3 with the alfa isomer precipitates so it does not further react to 2-isomer. Formation of
alfa is quick reaction driven kinetically, rearrangement from alfa to beta is slower and driven thermodynamically in a solvent in which the AlCl3
complex of alfa isomer is soluble (nitrobenzene).
|
|
|
Monoamine
Hazard to Others
  
Posts: 160
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
I used nitrobenzene as the solvent, since it is polar enough to dissolve the AlCl3 complex so the reaction can tend toward the thermodynamically
favoured 2-isomer. But I still think there could be a mixture because I ran the reaction at about 60C instead of -10C, w
Nitrobenzene's boiling point is only 8C higher than that of naphthalene, but naphthalene also sublimes at 80C. I've seen it sublime out of hexane and
THF before, but maybe it doesn't sublime out of nitrobenzene?
I guess I could do some TLC with pure naphthalene to check. Maybe it's also nitrobenzene?
In any case, do you
|
|
|
Monoamine
Hazard to Others
  
Posts: 160
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Actually, looking at these again I'm noticing that the brightest (orange) spot is actually still at the "start" line. Does this mean its too polar for
OH-silica stationary phases in general?
Like looking at the 40% EtOAc 60% hexane plate, there is a very narrow band of orange right at the solvent front and a very bright spot at the start
line (the blue is all over and even seems to have diffused back down. So the blue is super soluble in ethyl acetate, while the orange barely is? I
really don't know... gotta do more tests.
|
|
|
Monoamine
Hazard to Others
  
Posts: 160
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Aaaand... were back.
Ok I did TLC with pure naphthalene and with pure nitrobenzene to see if they could be constituents of the brown oil, which is the product of the
acylation.

From left to right: Naphthalene in pure hexane. Naphthalene in 40% EtOAc 60% hexane. Nitrobenzene in pure hexane. Nitrobenzene in 40% EtOAc 60%
hexane.

Wavelength 254nm

Wavelength 365nm
Well, I guess this conclusively that naphthalene is not in the final product. For one, the colour is more purple, but it also gets concentrated in a
narrow band in the 40/60 EtOAc/hexane system - it doesn't diffuse into it. It also can't be the orange fluorescing compound, because its colour is not
orange.
Nitrobenzene is a bit more tricky, because it barely fluoresces at the two wavelength. It's only really visible at 254nm and even there it's pretty
hard to see it. It's just a very subtle red/orange spot.
This means that there could possibly be some hidden under the other stuff in the original plates. But honestly I doubt it, because in the
original plates there is nothing orang/red that is absent at 365nm but can be seen at 254nm.
So the conclusion I would draw is that the final product does indeed consist of two naphthalene-propanone isomers. (Unless double acylation occured,
but I kind of doubt this since an excess of naphthalene was used and carbonyls deactivate the ring making further substitution unlikely).
The question then is, which isomer is which? Theoretically the 2-isomer should be more abundant, so I'd guess its the orange fluorescing one.
But one problem is also that with all the solvent systems that were tested there is never 100% separation. At best, there is a concentrated band of
orange at the solvent front with a concentrated spot of orange that did not move at all and the blue isomer diffused all over the place in between. So
I guess with the 50/50 EtOAc/hexane system the first and very last (if at all any) fraction from a column would be the orange substance and the rest
very dilute blue substance.

|
|
|