DrDevice
Hazard to Self
 
Posts: 74
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
What's happening with my esterification and o-methylation?
Hi all,
So, I've been trying to obtain the methyl ester of ferulic acid, then o-methylating the result:
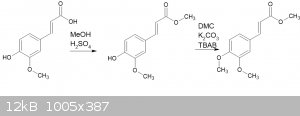
This should be a reasonably straightforward process, but I'm getting confusing results.
For the esterification, I have used two approaches. First is with methanolic HCl, warmed to 40C for a few hours. The other was with refluxing MeOH
with a few drops of sulphuric acid. The result after chromatographic cleanup was a yellow to yellow-brown oil. Methyl ferulate should be a white
solid. I figured I had some impurity that was preventing crystalization.
Both of these approaches yielded a compound with the same Rf on TLC (1:3 EtOAc:hexane).
I then used the substance for the o-methylation step. This involved dimethyl carbonate, potassium carbonate and TBAB, heated in a sealed vessel at
140C. I have used this process successfully in the past.
After work-up of the result, I obtained a yellowish solid with a MP of 62 - 64C - the exact melting point range of methyl ferulate. But the
TLC spot Rf for this substance was quite different to the Rf from the first "ester".
So I have no idea what's going on. A reaction is occurring each time, but whats happening at the first step, and how am I ending up with what I
*think* is the methyl ferulate at the second step??
BTW, I verified that the starting material was ferulic acid by MP.
Any suggestions?
|
|
|
Syn the Sizer
National Hazard
   
Posts: 591
Registered: 12-11-2019
Location: Canada
Member Is Offline
|
|
One concern I have is the double bond. I made a similar mistake when making methyl sorbate which has 2 double bonds. Alkenes run the risk of
hydrolysis in aqueous H2SO4 and you could end up with all sorts of products.
Another concern is something I have heard can happen in an ASA synth if not using Ac2O and trying to form the ester traditionally is that it could
form an ester with the phenolic group of another ferulic acid molecule.
One idea that might work but you need to find a suitable solvent, I tried similar with potassium sorbate and MeI in acetone, but it didn't work well.
But you may be able to form a sodium salt of the acid and react with MeI, this may also work with the phenol group too.
Another route to the initial ester could be to form an acyl halide and react with the alcohol.
|
|
|
|