Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Help me choose a barium salt
I'm considering looking at barium,
Which of the following would you choose for a useful source of barium?
1) >99.6% BaCl2.2H2O
2) >99.0% BaCO3
3) >98.0% Ba(OH)2.8H2O
4) xxxxx% Ba(NO3)2
#) relative cost of barium ion: major impurities
1) 1.00: Sr, Ca, K 0.05% each
2) 1.20: Sr 0.1%
3) 1.57: BaCO3 1%
4) 1.17: unknown - pyro grade
So, which would be 'best' as my sole source of Barium?
I'm thinking of the chloride due to purity, solubility and cost,
but the nitrate is available 250g min. with the others being 500g,
soluble and maybe a little pyro-fun,
but I'm concerned about the purity.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
The hydroxide is probably the simplest to make other barium compounds. Probably the most versitile, and so that woulkd be my choice.
|
|
|
MadHatter
International Hazard
    
Posts: 1332
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Barium salts
The chloride looks like your best bet for solubility and cost if the 0.15% total
impurities don't produce problems. Metathesis reactions with sodium salts should
crash out the barium equivalents of the other 3.
In pyrotechnics barium produces a green flame.
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
You also might want to look at moles of barium per dollar.
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
I think Ba(OH)2*8H2O is the best because of all other salts you can make from it but making 98% pure Ba(OH)2*8H2O is not trivial.
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 195
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
i would get the chloride, since you want to use it for pyro you dont want any trace potent contamination to ruin the color, like sodium
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I wouldn't.
I'm fairly sure that BaCl2 goes damp.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2656
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
A lot really depends on the usage. All have some uses in chemistry, but many uses require dry material, I think the carbonate is the easiet to keep
dry. The nitrate is fun to use to color fire in many ways. Of course boric acid is a safer source of pyro green, in theory.
But I might just go with the cheapest source for now, unless I had a clear need for one salt.
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I've had some BaCl2 since 2015 that's still dry, loose crystals, and most of that time it was
stored in a very humid environment. Definitely not super hygroscopic like MgCl2 or CaCl2.
|
|
|
Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Quote: Originally posted by teodor  | | I think Ba(OH)2*8H2O is the best because of all other salts you can make from it but making 98% pure Ba(OH)2*8H2O is not trivial.
|
Please enlighten me a little.
@ unionised : The relative costs are by weight of Ba, which is the same as by moles.
Looks like the hydroxide wins.
Thank you all.
I may make the purchase after I see what "neat things" are done by Antiswat if within my budget and abilities.
https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
(which is what peaked my interest in barium.)
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Kobold vor NH4
Hazard to Self
 
Posts: 56
Registered: 27-8-2019
Location: The sewer underneath your house
Member Is Offline
|
|
I would also recommend Ba(OH)2 as you can easily make other salts of Barium in one step most of the time, and there is no effervescence like on how
BaCO3 fizzes.
Less bubbles = less tiny droplets being sprayed into the air for you to breath in
"I don't need no excuse for being what I am"
-----Frank Zappa
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
The nitrate crystallizes nicely enough for that to be pointed out.
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
It's very hygroscopic and its solution very actively reacts with CO2 in the air. That's why getting of dry pure crystals of Ba(OH)2*8H2O is hard. But
this property is also actively used in many inorganic preparations of pure compounds - Ba(OH)2 is used in excess and then bubbling CO2 allows to
eliminate barium ions before filtering/crystallization. So, this is useful compound and in many preparations it has advantage over barium salts due to
the named property.
Also it could be used to prepare carbonate-free solutions of NaOH and KOH.
[Edited on 15-2-2022 by teodor]
[Edited on 15-2-2022 by teodor]
|
|
|
Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Thank you
I'm not at such an advanced level yet, and may never be.
(analysis of products is my main weakness and frustration)
But it's good to be pepared.
One more;
I doubt that I will use 500g of barium hydoxide quickly,
So
How should I store barium hydroxide for years/decades?
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
It depends on the source of the material. I would go for the BaCO3. This dissolves easily in all kinds of acids. But ... BaCO3 often is so-called
pottery grade material, which contains quite some sulfide, which is a very objectionable impurity, and which is hard to remove. I once made BaCl2 from
pottery grade BaCO3, but it was very hard to get rid of the sulfur (the sulfide forms very fine particles of sulfur on oxidation, e.g. from oxygen in
air).
If the BaCO3, however, is lab grade material, then I definitely would go for that. It has a very high relative amount of Ba, while the Ba(OH)2.8H2O
contains a lot of water.
From BaCO3 you can easily make other barium salts. Simply add a slight excess of the carbonate to dilute acid, heat a while and allow the excess BaCO3
to settle at the bottom. The resulting liquid then contains an almost pure solution of the barium salt, which easily can be obtained by letting the
solution slowly evaporate. In this way, you can make BaCl2.2H2O and Ba(NO3)2 easily.
I would not go for pyro-grade Ba(NO3)2. I have some of that, but it is rather impure and not that easy to purify, due to the limited solubility of
Ba(NO3)2. You need to dilute a lot, before all of it dissolves. Pure Ba(NO3)2 is better, but still somewhat limited. BaCO3 gives you more flexibility
(at the cost of a little bit more work).
BaCl2.2H2O has chloride ions, which coordinate to many transition metal ions, which can be annoying in some experiments. It is a useful chemical, but
converting the chloride to another barium salt is not that easy. So, with BaCl2.2H2O you buy convenience in direct use, but at the cost of some
flexibility.
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
I will show you. The hydroxide is already 2 years in this bottle. I don't see it absorbing more water, so I think this type of bottle is OK for
storage.

The second container is with a pottery-grade carbonate. I prefer to use that if I don't need the special properties of Ba(OH)2. But getting visible
pure BaCl2 from it requires 3 recrystallizations and a fume hood. So if I could find a good deal I would buy chemical pure BaCO3 as an addition to my
Ba stock.
By the way, I forget to mention one more interesting property of Ba(OH)2. Mixing 2 parts of solid Ba(OH)2*8H2O and 1 part of either NH4Cl or NH4NO3
creates a "cold pack" - the temperature drops to -30C - -25C in 2 mins. The waste would be BaCl2 or Ba(NO3)2 depending on the ammonium salt. I didn't
try it by myself but the experiment is described in the book "Chemical Demonstrations" (Shackhashiri), Vol. 1.
|
|
|
MidLifeChemist
Hazard to Others
  
Posts: 192
Registered: 4-7-2019
Location: West Coast USA
Member Is Offline
Mood: precipitatory
|
|
Where exactly (which company) are you going to purchase the barium salt from?
[Edited on 2/16/2022 by MidLifeChemist]
|
|
|
Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
https://shopee.com.my/product/441485525/8376315289?smtt=0.16...
https://shopee.com.my/product/441485525/9176314869?smtt=0.16...
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
...and it's obedient...a solution of barium nitrate which was quite yellow (perhaps micrograms of Fe?) was boiled down some and cooled, giving the 18
g of precipitate shown here, 95% of which was in the form of 2 almost but not quite perfect pyramids with sides exactly 1 inch long. The pyramids are
pure white and the rest is slightly yellow, and almost all of the yellow is gone from the mother liquor. The pyramids are made of crystals of the same
size as the yellow crystals, they're just stacked into pyramids and mortared together.
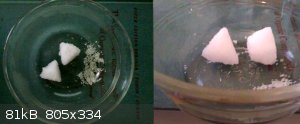
Quote: Originally posted by woelen  | I would not go for pyro-grade Ba(NO3)2. I have some of that, but it is rather impure and not that easy to purify, due to the limited solubility of
Ba(NO3)2. You need to dilute a lot, before all of it dissolves. Pure Ba(NO3)2 is better, but still somewhat limited. BaCO3 gives you more flexibility
(at the cost of a little bit more work).
BaCl2.2H2O has chloride ions, which coordinate to many transition metal ions, which can be annoying in some experiments. It is a useful chemical, but
converting the chloride to another barium salt is not that easy. |
BTW the nitrate has better solubility characteristics than the chloride, yet it can be made from the Na salt and the chloride, and the hydroxide can
be made from either.
|
|
|
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
well depends on what your uses are, i know you mean versatility- i just ordered 250g barium chloride, no need to mess around with acids but in case
you would want to use it to produce.... potassium hydroxide, as per Ba(OH)2 + K2SO4 = BaSO4 + KOH
well the barium hydroxide is kinda difficult to make yourself, or seemed so last i looked into it..
Ba(NO3)2 + Ca(OH)2 = Ba(OH)2 + .. no.
in case you dont just want it for precipitations the hydroxide is the most interesting, and can also be used for endothermic reaction- cant quite
remember what temperature it reaches, but def below comfort zone. for just basic use i'd go with barium chloride. barium sulfate can be achieved a bit
cheaper and you can Na2CO3 that in sol to get an amount of BaCO3 and this is also a way of recycling it from ppt reactions
again depending on use, the impurities could make a difference, for my uses it has zero impact. one cool use would be precipitating out barium
ferrate.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
The hydroxide is easy to precipitate with its very good solubility profile, the solution is much less reactive to CO2 when cold, and the octahydrate
crystallizes nicely.
|
|
|
Sulaiman
International Hazard
    
Posts: 3554
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Just ordered, thanks for all of the advice

CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|