MarangaMan
Harmless

Posts: 3
Registered: 11-12-2018
Member Is Offline
|
|
Advice for fractional distillation?
Hello,
I am trying to tackle a problem with a fractional distillation setup.
The story: I am making anhydrous food-grade ethanol for a future project. In the process, I am experimenting with methods to obtain food-grade ethanol
including fractional distillation from the cheapest box wine etc. The last method I tried was fractional distillation from a weak sugar wash.
I made about 10 L of sugar wash containing, in the end, about (I expect) 4 % ethanol. I then purified the wash by fractional distillation.
My setup: A 2L boiling flask filled with approx. 1600 ml sugar wash, then a 600x30 mm Vigreux column, and after that, an elbow and a condenser. I am
monitoring the temperature at the top of the column with a lab-grade digital thermometer. The 2L heating mantle that I am using has only four
settings: off, 125 W, 250 W and 500 W.
My goal: At this point, I am NOT attempting to obtain EtOH/water azeotrope, I am just trying to reduce the volume of the EtOH/water as much as
possible for final fractional distillation on a 1000/30 mm packed column with a distillation head to obtain the azeotrope and then drying that with
CaO.
The problem: When attempting the fractional distillation at the lowest setting of the heating mantle, the sugar wash is boiling, however, at the top
of the column, the temperature is gradually rising after distilling approx. 3 of the 4 % EtOH in the sugar wash. I am collecting whatever comes out,
starting at >85 % EtOH and ending at 10 % EtOH, the temperature at the top of the column gradually rises from 78,5 to 90 °C, the dripping is
slowing and slowing down and I never manage to deplete the EtOH from the wash even after 3 h process. If I do anything - insulating the flask,
increase the wattage of the mantle, anything, the temperature at the top of the column jumps to 99,2<t<99,5 °C, so the column is full of vapor
and I am essentially doing a simple distillation with extra steps. Guessing from this temperature, the wash still has 0,5-0,8 % EtOH which I struggle
with getting out, losing 20 % of total EtOH produced by my lovely yeast.
Is it possible to deplete the EtOH in the wash completely without endlessly waiting? What is my problem? I never get the textbook drop of temperature
after depletion of the low-boiling substance, the temperature just gradually increases. Is it possible with my setup or some modifications to it,
obtain the EtOH depletion and temperature drop with EtOH/water mixture? From what I see, It would seem I would have to constantly decrease and
decrease the heat supplied or have a longer and longer column, further slowing down the distillation, in order to, in an infinite amount of time,
reach the depletion of EtOH.
My guess would be that the problem is the low concentration of EtOH in the wash (however I had the same problem with distilling the boxed wine -
always got 10,2 % out of the listed 11 % EtOH).
I am just curious why I never manage to completely deplete all of the EtOH, since for me it seems the depletion time would limit to infinity.
Thank you all for discussion and advice!
EDIT: In theory, wouldn't I be able to obtain the depletion/temperature drop, and not gradual temperature increase, on a column with a finite amount
of theoretical plates, only in the case that the two separated compounds did not create azeotropic mixture?
[Edited on 27-2-2022 by MarangaMan]
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Not sure if this helps:
I'd start with a higher sugar concentration and get a higher ABV to start with but... since you are here already. I suggest setting up a simple still-
without a column- and boiling most of the alcohol out. That should give you a smaller volume of higher grade material to fractionate.
Sugar is cheap; yeast work for free.
If you are really lucky, the weather lets you freeze out some water before you even start spending money on electricity for the hotplate.
So you can sacrifice yield for convenience.
|
|
|
markx
National Hazard
   
Posts: 645
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Well....first of all it is not really possible to deplete the wash completely. It is much like trying to achieve the 0K setpoint, so just abandon that
idea and accept that there shall be losses.
For the second point, just adding a packed column inbetween the boiler and condenser does not really constitute a fractional distillation setup. For
true fractionation capability one needs adjustable reflux control: the vapors rising up the column must be condensed and led back down the way they
came with a small portion being diverted to output and advisably through an aftercooling unit to avoid boiling hot product. The amount of that portion
has to be adjustable by appropriate means like a needle valve e.g. The smaller the portion taken as output, the better the separation ability.
The core takeaway message here is that a column DOES NOT fractionate without reflux and without a proper reflux ratio. One can have a ten meter high
packed monstrosity, but if the upper end is only fitted with a pipe going to the output condenser, then it is all in vain and just a waste of space.
Might as well run a simple distillation setup and skip the column.
So why do textbooks describe a setup of the following configuration as a fractional distillation setup: boiler>column>output condenser? Because
at a very limited window of input power this setup can function like a reflux regulated fractionation system. Heat losses through the walls of the
column condense just the right amount of vapor and create enough reflux to operate the column with optimal fractionation ability. The problem is that
this window is narrow and shifts depending on the composition of the contents of the boiler. Which do inevitably change as one collects product at the
output and depletes the wash. In other words the setup is despair inducingly sensitive and almost uncontrollable. The only knob to play with is the
amount of power supplied into the boiler. Increase heat input a little and reflux ratio drops down taking fractionation ability with it....decrease it
a little too much and nothing comes over, as all vapor is condensed and turns to reflux inside the column without reaching output condenser.
That is exactly what you observed happening when insulating the column or increasing power to the boiler: reflux ratio dropped, because heat losses
decreased due to insulation or heat losses decreased vs. total heat supplied. Both lead to lower reflux ratio and lost fractionation ability, signaled
by an increase of temperature rise at the top of the column.
Long story short: if you want to properly fractionate, then reflux control stage is a must. It allows you to achieve stable operation below the
flooding point of the column and with minimal disturbances from outside factors like heat losses. Right now heat losses are the ones governing the
setup.
As a sidenote....commercial wines are not a very suitable starting material for ethanol production off the shelf. They contain sulfites and it shall
break down during distillation with the release of sulfur dioxide. It shall contaminate the product and is a nuisance to get rid of.
Exact science is a figment of imagination.......
|
|
|
Rainwater
National Hazard
   
Posts: 799
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
You can purchase liquor, which is 95% ethonol.(190 proff)
If you want to do it yourself, then temperature control is a must. I dont think a 4 point switch is going to work for this. Heres what i do. Forget
about temps.
I didn't really use a thermometer except to see when the distillation was finished.
As the solution temperature rises and reaches the first fraction, vapor will begin to rise up the column. Adjust heating down to prevent passing this
fraction.
Keep turning the heat down until the vapor does not make it all the way up the column and remains stable.
At this point, the column is refluxing 100%.
Note the highest point the vapor reaches in your column.
Turn up the heat by a small amount, then wait. Watch the vapor in the column rise higher
Only when the vapor has stopped rising do you continue
Adjust heating in such small increments that there is a noticeable delay in vapor rising,
Keep turning the heat up and waiting until the vapor reaches the highest point it can reach.
Once it gets to the top of the column, the thermometer temp starts to rise.
The fact that the head temp is warming up means that vapor is reaching it. Depending on your setup, the temp you see is not a defiant vapor temp. That
doesn't affect what you're doing. You're watching the vapor and providing just enough heat to let it barely reach the condincer column.
Your goal is to keep your column refluxing 99.99% of the vapor.
The balance is time. If you reflux 99%, your distillation will go faster but be less pure; slower will give you more purity but take longer.
As the concentration of your target decreases in solution, the boiling point will begin to change. You will notice that the vapor will no longer reach
the top of the column. Very slowly and in small increments, adjust heating up. Again, just enough for the vapor to reach the top.
Good luck
"You can't do that" - challenge accepted
|
|
|
Sulaiman
International Hazard
    
Posts: 3555
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
99.9% reflux is 1000:1 and 99% is 100:1
good in theory - impractically slow and expensive in reality.
Although possible,
I advise against going for anything near azeotropic using a Vigreux column.
You will end up performing many many many distillation runs.
I suggest either a (reasonably) good fractionating still
(seriouslly, quite a project)
or buy the best ethanol that you can get and start from there.
.........
At what I consider a moderate rate,
my typical Chinese 300mm Vigreux adds between one and two theoretical plates.
Filled with 3mm glass balls about three to four (with a reflux condenser),
a plain 300mm column with 3mm balls gives four to five with reflux around 3:1
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
MarangaMan
Harmless

Posts: 3
Registered: 11-12-2018
Member Is Offline
|
|
Thank you all of you for your answers and advice.
At this point, I am done with distilling the wash, so the advice that I asked for is for future runs, to improve. The theory of fractional
distillation is clear to me, including that a high reflux ratio is necessary for proper separation, but very hard to control with a heating mantle
with only 3 power settings. I am still pretty sure that I did pretty well with what I had and the number of theoretical plates/separation was decent
since I was turning a 4 % wash into > 85 % EtOH with the combination of my mantle on the lowest heat and careful manual cooling of the top of the
column with a lukewarm wet towel to achieve higher reflux ratio  . .
As I said, my main goal at this point was to reduce the volume of the EtOH/water with the yield as high as possible - again I say that this is not the
final part of the EtOH purification (the setup I am using = limited temperature control, no distillation head is closer to a laboratorified moonshiner
potstill than proper setup for fractional distillation) so I do not aim for the azeotrope, this step will come later - I am very well able to do
proper continual fractional distillation on a setup with a 1000/30 mm packed column and a distillation head - but the largest heating mantle with
precise temperature control that I have is for 1L flasks and I refuse to process 10L of wash in 750 mL steps  The one thing that comes to my mind that I could do to achieve better temperature control would be using a stirred
mineral oil bath on a lab hotplate/mixer instead of a mantle, but so far, I tried to avoid oil baths if I could use a simple heating mantle. The one thing that comes to my mind that I could do to achieve better temperature control would be using a stirred
mineral oil bath on a lab hotplate/mixer instead of a mantle, but so far, I tried to avoid oil baths if I could use a simple heating mantle.
My main question is, is it possible to improve any of it (yield/time/work) with my setup? From your answers and what I'm deducing I conclude that for
me, I struck the balance between the volume reduction, EtOH yield and my convenience (the column temperature maintenance was a tad bit of a pain  ). I will therefore deal with the fact, that the 0.5-0.8 % EtOH just goes to waste -
getting the last few drops of EtOH would be very uneconomical and time-consuming for me with the best way of improving my % yield would be starting
with a material with higher EtOH content. ). I will therefore deal with the fact, that the 0.5-0.8 % EtOH just goes to waste -
getting the last few drops of EtOH would be very uneconomical and time-consuming for me with the best way of improving my % yield would be starting
with a material with higher EtOH content.
I know that I can buy 95% food-grade ethanol for a pretty good price (1L of 96.4% EtOH for 23,70 $ when converted to USD) and if I wanted to be
effective, I would use that, but again, it is my hobby and this (including the problem-solving part) is just a part of the fun for me  If I used high-proof alcohol as the start of the process, I wouldn't have to do any
of the fermentation, preconcentration and purification steps and all I would need to do was reflux with CaO and simple distillation, so in the end, I
would be paying more for the high-proof EtOH to take the majority of the fun away If I used high-proof alcohol as the start of the process, I wouldn't have to do any
of the fermentation, preconcentration and purification steps and all I would need to do was reflux with CaO and simple distillation, so in the end, I
would be paying more for the high-proof EtOH to take the majority of the fun away 
Again, thank you all 
|
|
|
Rainwater
National Hazard
   
Posts: 799
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Quote: Originally posted by unionised  | Not sure if this helps:
If you are really lucky, the weather lets you freeze out some water before you even start spending money on electricity for the hotplate.
|
So,you can place a large container in the freezer and wait until it starts to freeze.
Before the ice gets too thick, bust it up and remove the solids. Top off the container as needed, repeat.
As the consentration of alcohol increases, the melting point of the fluid will decrease, and your standard freezer should be able to reach - 10c.
That should help you get consentrated up to.... 1 sec
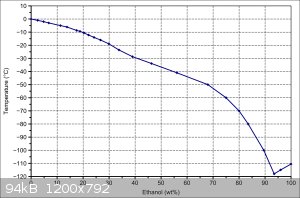
Around 20%
If you have access to a colder environment, then all they better.
Dry ice is another option. (-109c)
I've never tried these, but I have left beer in the snow and come back to a nasty shot,
"You can't do that" - challenge accepted
|
|
|
Sulaiman
International Hazard
    
Posts: 3555
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Temperature control is good for an oil bath,
for a heating mantle adjustable constant heating power is best.
You could use a dimmer/scr controller external to your mantle to adjust from zero to maximum, or zero to 250W, 500W etc.
not suitable if the mantle has a stirrer (switched on)
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
A blocking diode would also work, cutting the power by 50%.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
If you've got a 1 meter column you are talking serious enough equipment that you really ought to get a variable reflux head.
As Markx said, it is vital for good results.
This is especially true if your big column is jacketed.
The one pictured is a popular and cheap pattern, but works well.
I wouldn't bother with an electromagnetic head for just separating alcohol, but they sure do the job for higher reflux rates and more difficult
materials.

Edit: a 100:1 reflux ratio is, I think, excessive.
10:1 would be more suitable for ethanol.
100:1 is more for fractionating xylene isomers or separating other materials with 2 or 3 degree BP differences.
[Edited on 6-3-2022 by SWIM]
|
|
|
markx
National Hazard
   
Posts: 645
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Aye....such glass contrapulations intrinsically tend to have a rather short half-life  I can almost see it wanting to break up just out of pure contempt towards the poor master distiller.... I can almost see it wanting to break up just out of pure contempt towards the poor master distiller....
A more persistant solution might look something like this :

Exact science is a figment of imagination.......
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
I've used these for years without mishap.
My one broken one was broken in shipping.
Edit: although glass can break, it's nice having a head you can use for distilling corrosive materials as well.
[Edited on 6-3-2022 by SWIM]
|
|
|
Mateo_swe
National Hazard
   
Posts: 501
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
I have a 25L all stainless alcohole distiller with electric heating that takes 25L 20% fermented yeast/sugar mix.
It has a 8cm wide column about 1meter tall and it is filled with small ceramic rings.
This unit distills out over 90% ethanol that after dilution and active carbon treatment is much better than store bought vodka.
It self-regulates so no temp adjustments needs to be done, just plug and play.
Just make sure the cooling water is running to the stainless condenser.
Output temp is always a bit over 87 (before condenser) and stays there until the majority of the ethanol is distilled.
If i ever wanted to make anhydrous ethanol i would start with something like this and then try purify i further.
Distill fermented yeast/sugar at 20% and get >90%.
Dilute down to 40%, run thorough active carbon (use good carbon, important).
Then dilute again down to 20% and re-distill in the big distiller so one get cleaner ethanol ie. less impurities like other alcohols and fermentation
byproducts.
Then one have a good, pretty clean high % ethanol (i judge it food grade or drink grade+) for further purification with other methods like mol sieves
e.t.c.
|
|
|