alchemizt
Harmless

Posts: 36
Registered: 28-1-2019
Member Is Offline
|
|
How to analyse plant extracts with TLC
I made an alcohol extract (40% ethanol) with bark from a rare medicinal tree that grows in the jungle, and evaporated most of tge solvent, then
spotted it on a TLC plate and first visualised with UV of 254 nm:

Then put it in an iodine chamber:
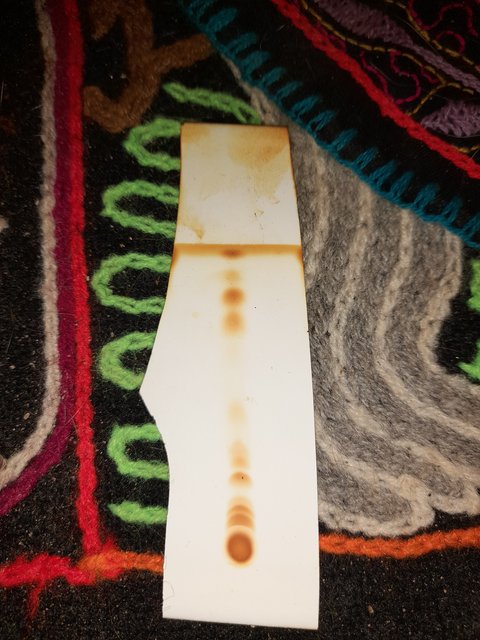
The mobile phase for this was 50:50 ethyl acetate : petroleum ether.
Here is a second plate I did with pure ethyl acetate:
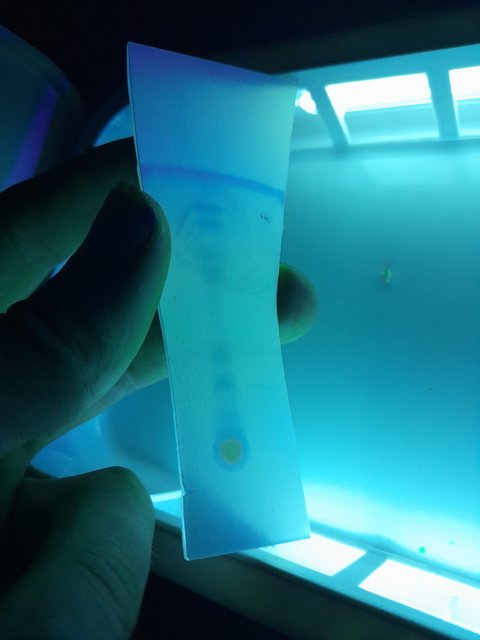
Firstly I have a few questions about how to read this:
1.) How many unique compounds would you estimate are on these plates?
2.) The plates look very similar for both mobile phases. What does this mean?
3.) Why is there a line at the top of the plate where the solvent line is? Is this a substance that is highly soluble in the mobile phase?
4.) And the big spot at the bottom where I spotted, is this compound(s) that are highly insoluble in the mobile phase?
5.) Some new spots appeared when stained with iodine, are these non UV active compounds or are they just more visible with the iodine?
Where would you go from here to find out more about what these compounds on the plate are?
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
Although I am suspicious of this rare "jungle" bark you are trying to work with... (sounds a bit similar to a rather "special" bark I have heard of) I
will avoid answering the questions directly and just help you with theory.
1.) No spoonfeeding answers. What do you think?
2.) What is the difference bewteen ethyl acetate and petroleum ether? Do you think that the hydrophobicity or dipole interactions could be affecting
how the components interact with the solvent system? If so, what happens to different functional groups (separate or in conjunction to one another)?
Why might these two solvent systems be so similar? Are they actually the similar, or are there two sets of contents you are mistaking as similar?
3.) Line at the top just indicates that a lot of contents moved at the speed of the solvent line, which likely indicates that you need a better
solvent system, or need to break the chromatography into separate runs.
4.) Well given the information I have so far provided, this is answerable, and it seems you have a good idea already of ehat caused it.
5.) Iodine is just a cheap developer so that we can clearly see what is a spot and what isnt. Its sometimes hard to see spots if they absorb uv light,
and sometimes even in uv light they are a pain. Iodine allows one to keep an "image" or "shadow" of the products. However this doesnt work for all
chemicals.
6.) Unless you are a professional chemist, which I am assuming you are not, and unless you have access to a GCMS or some equivalent, identification is
going to be super hard. Take it from pre 1900s chemists, they did this stuff by hand and by countless hours of reacting each component isolated until
they got a very crude structure, and even then it is hard. If you know the species, you can probably look up the breakdown of components in the
extract and use cross references to do so. But if you are also talking about separation post-chroma column fractioning, you need to be sure that all
fractions are isolates and do not contain more than 1 compound. This could take an amatuer months if not longer to do, if done properly. It took me
ages to get good at chromatography, even with my lab professor teaching me under direct guidance. If you wish to do so, then take your time and take
copious notes with lots of experimentation. Id suggest getting more of that bark if you want to get serious. Otherwise, it may be very difficult.
There is also the possibility of very crude separations if you know the compound of interest, but be careful, this is also sometimes difficult. Just
look to the forum posts about isolations from various substrates (black pepper corns, chiles, etc).
Also if this is drug related, its best you stop here and head to another forum, this stuff isnt tolerated here. Although I dont want to assume, your
lack of stating the scientific name of this special tree gives rise to a feeling of drug chemistry. I am personally indifferent to drug chem, it seems
cool in terms of building molecules, however you seem to be extracting from a bark, which if it is what I believe it is, could land you in some
serious issues depending on where you live.
EDIT - Most definitely drug extraction. You sir are looking for DMT and harmaline alkaloids... you said so by giving the name of the plant in your
older posts.
Exhibit A : http://www.sciencemadness.org/talk/viewthread.php?tid=158450...
Exhibit B : http://www.sciencemadness.org/talk/viewthread.php?tid=158450...
Listen, your post history gives it away big time. From saying that you got a BSc in chemistry, to extraction help for "alkaloids", and having no clue
about mixtures of solvents and chromatography, makes this scream drug chemistry. Go to the HIVE or the Vespiary, they have plenty of information. Drug
chemistry would get this beautiful site taken down, so please dont ruin it!
[Edited on 4-30-2022 by aab18011]
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
@aab18011:
This site is a lot more tolerant of drug chemistry than you think it is.
(unless there have been some big recent changes I missed. Anybody official care to set this straight?)
However you are spot-on about spoon-feeding.
Also you are spot-on about deceptive posts as they often indicate bad intentions and even worse indicate a poster who thinks we are a bunch of
freaking idiots here to be manipulated for fun and profit.
Thanks for checking his history and letting us know how full of crap he is too. That was very cool.
@alchemizt:
Your post is terrible, and shows not only zero understanding of what you're doing, but a total lack of any real attempt to figure it out or research
it.
I don't know crap about TLC, but my near total ignorance is still miles ahead of this post.
I am amazed that aab18011 went to as much trouble as he did to explain things to you.
Few people would be so considerate about a post like yours.
Do some research. Start with basic texts and lab manuals because there's no way you've got a chem degree. You can do it if you're motivated, but it
won't come easy and will take time.
And I wouldn't recommend taking this post to a place like the vespiary.
They are, if anything, less tolerant of crap like this and you'll be lucky if you get totally ignored instead of getting the spit-roasting you
deserve.
aab18011 was trying to help you, and believe it or not, so am I.
Trying to do chemistry for human consumption with your level of knowledge is like playing Russian roulette.
Start learning and you'll soon be able to ask questions worth answering.
This isn't rocket science, but unfortunately the main difference between rocket science and organic chemistry is just the size of the disasters when
you screw up.
You can still die, but you're less likely to take others with you.
That's no joke. we lose good, experienced people here. Probably more than we know about, but I could name a few I considered real friends who are now
taking the long dirt-nap.
Edit: not to mention others who are now missing various important bits and pieces that don't grow back, and some of those were real chemists with
Doctorates and years of professional experience.
[Edited on 30-4-2022 by SWIM]
|
|
|
alchemizt
Harmless

Posts: 36
Registered: 28-1-2019
Member Is Offline
|
|
Quote: Originally posted by aab18011  | | Although I am suspicious of this rare "jungle" bark you are trying to work with... (sounds a bit similar to a rather "special" bark I have heard of) I
will avoid answering the questions directly and just help you with theory. |
The plant is in the tabernaemontana genus. I beleive its tabernaemontana sananho but may be mistaking it with a related species.
I should have explained a bit beter when I wrote these questions, so as not to give the wrong idea. I think that there are 10 unique UV active
compounds appearing on the plates. The crescents I believe are formed because I didn't have a capillary to spot the sample, so I disturbed the silica
matrix a bit with the pin. I'm wondering can this situation make it appear like theres numerous compounds separating, when really its only one.
Quote: Originally posted by aab18011  |
2.) What is the difference bewteen ethyl acetate and petroleum ether? Do you think that the hydrophobicity or dipole interactions could be affecting
how the components interact with the solvent system? If so, what happens to different functional groups (separate or in conjunction to one another)?
Why might these two solvent systems be so similar? Are they actually the similar, or are there two sets of contents you are mistaking as similar?
|
Petroleum ether is made up mainly of short chain aliphatic hydrocarbons which don't have major dipole moments to solvate more polar functional groups.
Increasing the pet. ether would usually cause the components to spread differently, as the more polar constituents become less soluble in the mobile
phase but could be that the concentration of pet. ether needs to reach a certain threshold where the ethyl acetate becomes so dispersed that its
electronic interactions with the polar functional groups in the sample become negligent. The second plate might be a different spread of compounds
entirely, but it does look very similar.
Quote: Originally posted by aab18011  |
3.) Line at the top just indicates that a lot of contents moved at the speed of the solvent line, which likely indicates that you need a better
solvent system, or need to break the chromatography into separate runs.
|
This is what I thought. I expected the different solvent system to slow down the components of this line, but that didn't appear to happen. Or could
just be that lowering the polarity increased the solubility of another set of compounds in the sample.
Quote: Originally posted by aab18011  |
6.) Unless you are a professional chemist, which I am assuming you are not, and unless you have access to a GCMS or some equivalent, identification is
going to be super hard. Take it from pre 1900s chemists, they did this stuff by hand and by countless hours of reacting each component isolated until
they got a very crude structure, and even then it is hard. If you know the species, you can probably look up the breakdown of components in the
extract and use cross references to do so.
But if you are also talking about separation post-chroma column fractioning, you need to be sure that all fractions are isolates and do not contain
more than 1 compound. This could take an amatuer months if not longer to do, if done properly. It took me ages to get good at chromatography, even
with my lab professor teaching me under direct guidance. If you wish to do so, then take your time and take copious notes with lots of
experimentation. Id suggest getting more of that bark if you want to get serious. Otherwise, it may be very difficult.
There is also the possibility of very crude separations if you know the compound of interest, but be careful, this is also sometimes difficult. Just
look to the forum posts about isolations from various substrates (black pepper corns, chiles, etc).
|
I want to be able to narrow it down as much as I can myself before sending the samples to professional labs for GCMS, HNMR, IR spectra, all this.
Quote: Originally posted by aab18011  |
Also if this is drug related, its best you stop here and head to another forum, this stuff isnt tolerated here. Although I dont want to assume, your
lack of stating the scientific name of this special tree gives rise to a feeling of drug chemistry. I am personally indifferent to drug chem, it seems
cool in terms of building molecules, however you seem to be extracting from a bark, which if it is what I believe it is, could land you in some
serious issues depending on where you live.
|
Extracting capsaicin from chiles could be considered "drug related" as capsaicin has pharmacological properties, no? I live in the jungle and am
working on developing my ability to extracting interesting, unknown components of plants, with the intention of eventually being able to discover and
produce extracts that will be of benefit to humanity. It is "drug" chemistry I'm talking about because these plant extracts can be labelled as
"drugs". My last thread was about harmala alkaloids from a little known species of bsnisteriopsis vine, and these alkaloids are very interesting. Its
common knowledge in the jungle here that these vines have the ability to regenerate the brain.
This plant in this thread is known as a great doctor tree, capable of curing a wide range of physical and psychosomatic illnesses. I suspect this one
is full of neurotrophic factors too.
Quote: Originally posted by aab18011  |
Listen, your post history gives it away big time. From saying that you got a BSc in chemistry, to extraction help for "alkaloids", and having no clue
about mixtures of solvents and chromatography, makes this scream drug chemistry. Go to the HIVE or the Vespiary, they have plenty of information. Drug
chemistry would get this beautiful site taken down, so please dont ruin it!
|
If we were talking face to face about this, these ideas would be smashed in seconds. Theres a context that doesnt get conveyed through text on a
forum.
[Edited on 30-4-2022 by alchemizt]
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
I mean even the plant you have given in this thread contains ibogaine, which is schedule 1 in the US, which is pretty much a life sentence in the
clank.
But to actually address your questions as you have expanded, I am happy to help.
In terms of separation, your crescents are all different compounds. Take the photo of it and draw a dot at each center of the crescent. Count them.
This gives a good idea. In my lab at work, we work with porphyrins a lot and we tend to do what we call a "smear" technique where we dont dot the
reaction mixture but instead smear it in a nice thin line horizontally. This allows us to visualize better, however porphyrins are known to be colored
which makes it a whole lot easier. You can also try that, but be sure to let the applied extract mixture to dry on the plate first. You can even use
gentle heating.
You are more than likely going to need to mess with more solvents. Make a list of solvents from totally non polar to highest polarity. Then mess
around with mixtures. As long as you can get a nice separation, you can use the Rf values to determine the length of the chroma column needed. Its not
perfect by any means, but ive separated 120 compounds in one column before, which honestly I got lucky with that. It was just perfect conditions. Wont
happen again in my case haha.
I am assuming here, but Id say from experience that plant sometimes behave similarly even when a solvent is ommitted. So again, mess around and see
what you get. Try and use a longer tlc plate, you might be surprised with what some extra time and patience can yield you.
As for the solvent front issue, maybe try and decrease the height of the solvent at the bottom of the plate. Sometimes when you submerge the plate
such that the solvent system starts on top of the area in which you spotted the plate, you will get a nasty dissolution and will ruin the plate.
As for GCMS, use the chemists in Mexico. Its entirely legal to work with tryptamines there, and they are a skilled bunch. And yeah just keep working
on the separation.

I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
Mateo_swe
National Hazard
   
Posts: 505
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
There is no rules against drug related chemistry on this forum as long as it focuses on the interesting chemistry and are not plain drug cookery
instructions.
And this is the Beginnings section, one can expect beginner questions and posters that dont know how to efficiently search and look up the chemical
information they seek.
Do you think its inspiring and good for new chemistry hobbyists to be told to shut up, dont post, go away and come back when you already have learned
and have as you say questions worth answering?
Give him some good answers or info where he can find the answers instead of complaining.
Amazing how stupid some people are.
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
Quote: Originally posted by Mateo_swe  | There is no rules against drug related chemistry on this forum as long as it focuses on the interesting chemistry and are not plain drug cookery
instructions.
And this is the Beginnings section, one can expect beginner questions and posters that dont know how to efficiently search and look up the chemical
information they seek.
Do you think its inspiring and good for new chemistry hobbyists to be told to shut up, dont post, go away and come back when you already have learned
and have as you say questions worth answering?
Give him some good answers or info where he can find the answers instead of complaining.
Amazing how stupid some people are. |
Not to be rude, but he said he had a bachelors in chemistry and the questions he asked were a bit odd for a bachelors to ask. I mean I dont have my
degree, but the grads in my lab have shocked me with how smart they are. Maybe I have a wonderful batch to work with... who knows. Maybe I was quick
to judge, as I am still learning the ropes on SM. Also, I have read a lot in detritus, which is what made me think that this kind of stuff isnt
allowed. My appologies.
If he were talking to me face to face, Id have no problem explaing to then what I know. And now that I know it isnt bad to talk about this stuff, I
will be more open.
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
alchemizt
Harmless

Posts: 36
Registered: 28-1-2019
Member Is Offline
|
|
Quote: Originally posted by aab18011  | | I mean even the plant you have given in this thread contains ibogaine, which is schedule 1 in the US, which is pretty much a life sentence in the
clank. |
As far as I know, this plant does not contain ibogaine, it contains some other alkaloids that are present in iboga as these plants are im the same
family. If it contained ibogaine, I would assume its in a very small quantity. In my country ibogaine is not prohibited as far as I know.
Quote: Originally posted by aab18011  |
In terms of separation, your crescents are all different compounds. Take the photo of it and draw a dot at each center of the crescent. Count them.
This gives a good idea. In my lab at work, we work with porphyrins a lot and we tend to do what we call a "smear" technique where we dont dot the
reaction mixture but instead smear it in a nice thin line horizontally. This allows us to visualize better, however porphyrins are known to be colored
which makes it a whole lot easier. You can also try that, but be sure to let the applied extract mixture to dry on the plate first. You can even use
gentle heating. |
Thanks. I really just wanted to confirm that they are separate compounds, to understand if there might be situations where this crescent formation
might make one compound appear as multiple compounds. I know how to resolve these dots by altering the solvent system. I usually run the plate again
time in solvent system that doesn't elute the compounds very well, and repeat if needs be, slightly altering the system each run. Eventually they will
separate.
Quote: Originally posted by aab18011  |
You are more than likely going to need to mess with more solvents. Make a list of solvents from totally non polar to highest polarity. Then mess
around with mixtures. As long as you can get a nice separation, you can use the Rf values to determine the length of the chroma column needed. Its not
perfect by any means, but ive separated 120 compounds in one column before, which honestly I got lucky with that. It was just perfect conditions. Wont
happen again in my case haha.
|
When I have a complex mixture, I usually run one TLC plate multiple times until I find a few solvent systems in a specific order which spread all the
compounds well, sometimes some of the compounds have to run off the plate first. So when I run the column, only a portion of the compounds elute first
pass, then the rest come out with a different solvent system.
120 compounds in one column is pretty incredible alright, out of curiosity, what exactly was the mixture and what was the solvent system that
separated it?
|
|
|
alchemizt
Harmless

Posts: 36
Registered: 28-1-2019
Member Is Offline
|
|
Quote: Originally posted by aab18011  | Quote: Originally posted by Mateo_swe  | There is no rules against drug related chemistry on this forum as long as it focuses on the interesting chemistry and are not plain drug cookery
instructions.
And this is the Beginnings section, one can expect beginner questions and posters that dont know how to efficiently search and look up the chemical
information they seek.
Do you think its inspiring and good for new chemistry hobbyists to be told to shut up, dont post, go away and come back when you already have learned
and have as you say questions worth answering?
Give him some good answers or info where he can find the answers instead of complaining.
Amazing how stupid some people are. |
Not to be rude, but he said he had a bachelors in chemistry and the questions he asked were a bit odd for a bachelors to ask. I mean I dont have my
degree, but the grads in my lab have shocked me with how smart they are. Maybe I have a wonderful batch to work with... who knows. Maybe I was quick
to judge, as I am still learning the ropes on SM. Also, I have read a lot in detritus, which is what made me think that this kind of stuff isnt
allowed. My appologies.
If he were talking to me face to face, Id have no problem explaing to then what I know. And now that I know it isnt bad to talk about this stuff, I
will be more open. |
Chemistry is big field, everyone has aspects that they are lacking in understanding. Believe it or not, I have so much experience with porphyrinoids
that I could probably walk into your lab and know exactly what I'm doing.
|
|
|
|