Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
OTC Vanilin
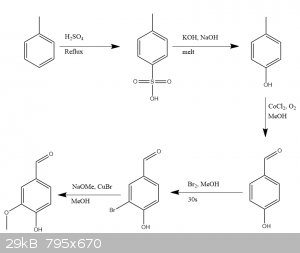
I've been pretty bored lately, so I decided to take on a larger project. I'm gonna try to produce vanillin from toluene. The full scheme is below. The
first two steps are shit straightforward, but from there on it gets pretty tough.
1)The oxidation reference is Autoxidation ofp-cresol top-hydroxy benzaldehyde using CoCl2, CoAPO-5, and CoAPO-11. URL. This seems fine, except they end up with a mix of p-hydroxy benzyl alcohol, p-hydroxy benzaldehyde, and p-hydroxybenzoic acid. Removing the
acid should be straightforward enough, just dissolve crude in DCM and then rinse with sodium bicarb solution. However, when using CoCl2 as the
catalyst, they report:
| Quote: |
80% in the case of CoCl,, by-products being p-hydroxy benzylalcohol
(p-HBAlc; 5%) and p-hydroxy benzyl methylether (p-HBME; 10%).
|
They measured these using HPLC, which isn't an option for me, so I'm trying to find a different separation method. Input on this would be appreciated.
2) The other issue is the need for sodium methoxide in the final step. The final two steps are from this citation: Vanillin Synthesis from
4-Hydroxybenzaldehyde. URL. I'm thinking about trying to get away with magnesium methoxide since I can buy magnesium shavings OTC as a firestarter, and have used it to
make methoxide for nitro-azo reductions in the past. I would think this would work. Happy for input on this. I just prepared the p-TsOH last weekend,
will update when I do the cresol step.
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2693
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
That oxidation looks pretty awesome. Shame it isn't general. The graphs mostly discuss the heterogeneous cobalt catalyst but you can see from Fig. 6
that the cobalt loading is about the same with CoCl2 -- less than 0.5%! The paper says 338 K which is refluxing methanol... and for 20 hours, so get
some nice coffee 
Your steps aside from the oxidation are pretty much the standard for this procedure. The Ullmann reaction on 3-bromo-4-hydroxybenzaldehyde mirrors a
similar well-known reaction on 5-bromovanillin to syringaldehyde which has been performed many times; you may find it helpful to consult resources
describing the latter reaction. I am not convinced that the bromination of p-hydroxybenzaldehyde will be complete in 30 seconds, but I don't think
that matters too much — nobody moves that fast.
| Quote: | | I'm thinking about trying to get away with magnesium methoxide since I can buy magnesium shavings OTC as a firestarter |
I would also consider KOMe from K2CO3 and MeOH. Not as easy as Mg(OMe)2 but easier than sodium or the NaOH method (K2CO3 is much easier to handle than
'dry' NaOH).
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Interesting. I'm gonna try out the whole alkali fusion step soon. I will report on how that goes. Maybe, just maybe, if my boss lets me, I might get
an IR and NMR of my product
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Mateo_swe
National Hazard
   
Posts: 505
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
Would that cobalt oxidation in methanol work on other aromatic substrates to make the corresponding aldehydes?
Toluene to benzaldehyde etc., the yields doesnt seem so bad.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2693
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The mechanism in the paper seems pretty specific to cresols, and particularly to o- and p- cresol which admit quinone-like intermediates. In short, I
wouldn't bet on it.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
I would doubt it works for normal toluene, but it probably would work for nitrotoluenes, since those can form the quinone-esque intermediate.
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|