| Pages:
1
2 |
Claisen
Hazard to Self
 
Posts: 70
Registered: 10-12-2010
Member Is Offline
Mood: No Mood
|
|
A small doubt-
Is the given compound chiral (please provide your reason too):

[Edited on 5-4-2011 by Claisen]
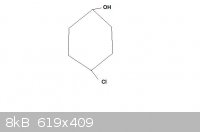
[Edited on 5-4-2011 by Claisen]
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
It is possible for some compounds to be cryptochiral, a chiral centre exists but the optical rotation is so weak that it cannot be measured but the
chirality may be exhibited in other ways;
http://en.wikipedia.org/wiki/Cryptochirality
[Edited on 5-4-2011 by ScienceSquirrel]
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Quote: Originally posted by Claisen  | A small doubt-
Is the given compound chiral (please provide your reason too):

[Edited on 5-4-2011 by Claisen]
Quite simply it is achiral as it has a plane of symmetry running through C1 and C4
[Edited on 5-4-2011 by Claisen] |
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
It is not chiral, because it has no mirror images (no "chiral centers") or more precisely the mirror images are identical due to the plane of
symmetry. However, it has a stereogenic center and can have two diastereomers. You will not notice these until you try to depict the molecular
structure in three dimensions (unless you are already familiar with such isomerism). If you attempt some structure modelling, you will see the
exocyclic groups can be in either trans or cis relation. Thus, the compound can have two stereoisomers (these are in diastereomeric relation). But it
has no optical isomers (no enantiomers). The situation is alike to alkene double bond isomerism - stereogenic but achiral.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I'm going to step in here and suggest that the OP finds a copy of the "stereochemistry bible" by Eliel. http://www.amazon.com/Stereochemistry-Organic-Compounds-Erne...
They should have a copy available at the library. Alternatively, perhaps someone has it in ebook format. If so I'd be greatly interested!
|
|
|
Claisen
Hazard to Self
 
Posts: 70
Registered: 10-12-2010
Member Is Offline
Mood: No Mood
|
|
How do we determine the outer electron configuration of lanthanoids and actinoids (with just atomic number in hand)? Please explain me the method
giving example of Gd (atomic no:64).
|
|
|
Claisen
Hazard to Self
 
Posts: 70
Registered: 10-12-2010
Member Is Offline
Mood: No Mood
|
|
No one knows the method???
|
|
|
Claisen
Hazard to Self
 
Posts: 70
Registered: 10-12-2010
Member Is Offline
Mood: No Mood
|
|
After getting disappointed from my last question, I am posting another with some hope.....
The electron affinity values of elements A,B,C and D are respectively -135, -60, -200 and -348 kJ/mol. The outer electronic configuration of element B
is
a)3s2 3p5
b)3s2 3p4
c)3s2 3p3
d)3s2 3p2
According to my book, a large positive electron affinity means that the negative ion is very stable. Going by that, the anion of B will be most stable
so its outer configuration is 3s2 3p5. The answer given is 3s2 3p3. Is my book wrong or the answer?
Please help!
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
A large positive electron affinity suggests its not a favourable process, and so you'd expect to be disrupting a stable configuration. If you assign
all of the electron affinities to the configurations, you should find:
a)3s2 3p5 -348kJ/mol - making noble gas configuration - completion of electronic shell.
d)3s2 3p2 -300kJ/mol - making a stable half filled shell (3p3)
b)3s2 3p4 -135kJ/mol - not disrupting a stable configuration, but not making anything exceptionally stable either
c)3s2 3p3 -60kJ/mol - disrupting the half filled 3p shell (3p3 is a stable config, see Photoelectron spectrum of atomic nitrogen c.f. neighbouring
elements - can be rationalised with a little bit of thought).
I'll see if I can answer your question on the lanthanides in a bit when I've had time to remember them lol...
Edit: In the book "Lanthanide and actinide chemistry" by Simon Cotton, he gives a clear cut explanation of the electronic configurations of the atomic
lanthanides. You should be able to see the paragraph in question here: http://books.google.co.uk/books?id=SvAbtU6XvzgC&pg=PA9&a...
[Edited on 14-5-2011 by DJF90]
|
|
|
Claisen
Hazard to Self
 
Posts: 70
Registered: 10-12-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DJF90  | | A large positive electron affinity suggests its not a favourable process, and so you'd expect to be disrupting a stable configuration.
|
Read this - http://en.wikipedia.org/wiki/Electron_affinity
Chlorine has the highest positive electron affinity value. Does it meant that it turning to an anion is unfavourable?
I don't know how to thank you. That cleared a big trouble from my head. Thank you very much!!
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
| Quote: | | The electron affinity, Eea, is defined as positive when the resulting ion has a lower energy, i.e. it is an exothermic process that releases energy
|
(from wikipedia page you link to).
The more exothermic the process is (i.e. the more large and negative the energy change is (negative is when energy is given out)), the more stable the
resulting ion is. The data on the page seems to be written with the second definition in mind:
| Quote: | Alternatively, electron affinity is often described as the amount of energy required to detach an electron from a singly charged negative ion,[1] i.e.
the energy change for the process
X− → X + e− |
I'm sure you'll agree that this is confusing. In this regime, a large positive value reflects high electron affinity. This ishow they've presented
the data given, as I guess it means they do not need to include minus signs. Recall that from Hess's law, the reverse chemical process has the same
magnitude of energy change, just the sign is different.
|
|
|
Claisen
Hazard to Self
 
Posts: 70
Registered: 10-12-2010
Member Is Offline
Mood: No Mood
|
|
| Quote: | | The more exothermic the process is (i.e. the more large and negative the energy change is (negative is when energy is given out)), the more stable the
resulting ion is. The data on the page seems to be written with the second definition in mind: |
I am getting confused :|
You will agree that more positive is the value of electron affinity, greater is the ability of that element to form an anion. For example, Cl has
electron affinity value of 349 kJ/mol. It means that 349 kJ of energy is required to remove an electron from the Chlorine anion. Sign convention for
electron affinity is opposite to that of electron gain enthalpy.
In my question, out of the values given, -60 is the most positive value. So the resulting anion should be most stable. B should be assigned 3s2 3p5
configuration keeping that in mind as it will form a noble gas configuration on turning to an anion.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
You're missing the content of my above post. Electron affinity can be defined in two ways:
X + e- => X- Chloride is more stable than chlorine, so Ea is negative (exothermic, favourable process)
or
X- => X + e- Removal of electron from stable chloride to form less stable chlorine atom is undesirable, so Ea is positive (endothermic,
requires energy to do so).
It appears that the data you are given is using the first definition, yet the data on the wikipedia page is using the latter definition.
In your question, -60 is the most positive value, and so corresponds to the LEAST favourable process. For the electronic configurations given, this
corresponds to the disruption of the stable half filled 3s2 3p3 configuration by addition of an electron.
[Edited on 16-5-2011 by DJF90]
|
|
|
Claisen
Hazard to Self
 
Posts: 70
Registered: 10-12-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DJF90  | You're missing the content of my above post. Electron affinity can be defined in two ways:
X + e- => X- Chloride is more stable than chlorine, so Ea is negative (exothermic, favourable process)
or
X- => X + e- Removal of electron from stable chloride to form less stable chlorine atom is undesirable, so Ea is positive (endothermic,
requires energy to do so). |
It may be defined in both ways, but its sign must remain same in both the definitions other wise there is no use of defining it. According to
wikipedia and my book, electron affinity is defined by your second definition. Your first definition defines electron gain enthalpy. The only
difference between these two is of the sign. Electron affinity is positive for electron acceptors.
"A molecule or atom that has a positive electron affinity is often called an electron acceptor" - from Wikipedia
I may be wrong somewhere again because my knowledge is very limited. I am just telling what I read.
Quote: Originally posted by DJF90  | It appears that the data you are given is using the first definition, yet the data on the wikipedia page is using the latter definition.
|
I think there is a misprint of electron affinity instead of electron gain enthalpy in the question.
|
|
|
| Pages:
1
2 |