SnailsAttack
Hazard to Others
  
Posts: 163
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Magnesium chromate from electrolysis of stainless steel in magnesium sulphate solution
Recently I discovered that chromates can be synthesized as magnesium chromate by open-cell electrolysis of magnesium sulphate solution with stainless
steel electrodes, and I've been trying to figure out how this works.
The following facts are known:
- The cathode (negative electrode) exhibits the heaviest chemical wear and generates a substantial amount of orange gunk, perhaps iron oxyhydroxide.
- The anode (positive electrode) is the source of the magnesium chromate, as it can be seen to accumulate on the threads of the stainless steel bolt
being used as the anode.
 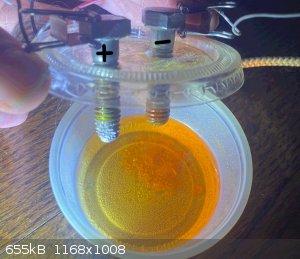
The anode appears heavily worn because it has previously been used as the cathode.
The only known electrochemical reactions are given below.
| Quote: |
// Anode (positive electrode) reaction (oxidation occurs)
SO₄²⁻(aq) + H₂O(l) -> 2H₂SO₄(aq) + O₂(g)
// Cathode (negative electrode) reaction (reduction occurs)
Mg²⁺(aq) + 2H₂O(l) -> 2Mg(OH)₂(aq/s) + H₂(g)
// Mixing reaction
H₂SO₄(aq) + Mg(OH)₂(aq/s) -> MgSO₄(aq) + 2H₂O(l)
|
Magnesium hydroxide doesn't appear to precipitate from the cathode, suggesting that most of it is redissolved by the sulphuric acid produced at the
anode.
The presence of sulphuric acid probably dissolves the iron in both electrodes:
Fe(s) + H₂SO₄(aq) -> FeSO₄(aq) + H₂(g)
Iron oxyhydroxide may be produced by oxidation of the iron(II) sulphate to iron(III) sulphate:
12FeSO₄(aq) + 2H₂O(l) + 3O₂(aq) -> 4Fe₂(SO₄)₃(aq) + 4FeO(OH)(s)
Iron oxyhydroxide may also be produced like this:
4Fe(s) + 3O₂(aq) + 2H₂O(l) -> 4FeO(OH)(s)
Some of the iron oxyhydroxide likely redissolves if excess sulphuric acid is present:
2FeO(OH)(s) + 3H₂SO₄(aq) -> Fe₂(SO₄)₃(aq) + 4H₂O(l)
Many of these reactions may have electrochemical analogs.
-
Iron(II) sulphate and magnesium chromate are likely incompatible in solution and ought to form iron chromate:
MgCrO₄(aq) + FeSO₄(aq) -> MgSO₄(aq) + FeCrO₄(s)
In spite of this, magnesium chromate does accumulate in solution. I'm not sure if an analogous reaction proceeds with iron(III) sulphate.
With all that said, I'm still not even sure how the chromate forms. Further testing will be done. Discussion is appreciated.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by SnailsAttack  |
12FeSO₄(aq) + 2H₂O(l) + 3O₂(aq) -> 4Fe₂(SO₄)₃(aq) + 4FeO(OH)(s)
Iron oxyhydroxide may also be produced like this:
4Fe(s) + 3O₂(aq) + 2H₂O(l) -> 4FeO(OH)(s)
Some of the iron oxyhydroxide likely redissolves if excess sulphuric acid is present:
2FeO(OH)(s) + 3H₂SO₄(aq) -> Fe₂(SO₄)₃(aq) + 4H₂O(l)
Many of these reactions may have electrochemical analogs.
-
Iron(II) sulphate and magnesium chromate are likely incompatible in solution and ought to form iron chromate:
MgCrO₄(aq) + FeSO₄(aq) -> MgSO₄(aq) + FeCrO₄(s)
In spite of this, magnesium chromate does accumulate in solution. I'm not sure if an analogous reaction proceeds with iron(III) sulphate.
With all that said, I'm still not even sure how the chromate forms. Further testing will be done. Discussion is appreciated. |
Have you any evidence you actually have formed some chromate, either in solution or as a precipitate?
If so, how did it form?
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 163
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by blogfast25  |
Have you any evidence you actually have formed some chromate, either in solution or as a precipitate?
If so, how did it form? |
I'm not sure how it forms, but I'm positive that there's substantial amount of soluble chromate. Turns orange in the presence of an acid, turns blue
and fizzes when I add peroxide.
Additionally:
| Quote: |
The magnesium chromate and magnesium sulphate can be isolated from any aqueous iron(II) sulphate or iron(III) sulphate by addition of ammonia
solution, which causes the iron to precipitate as insoluble iron(II) hydroxide and iron(III) oxyhydroxide, respectively:
FeSO₄(aq) + 2NH₃(aq) + 3H₂O(l) -> Fe(OH)₂(s) + (NH₄)₂SO₄(aq)
2Fe₂(SO₄)₃(aq) + 6NH₃(aq) + 4H₂O(l) -> 2FeO(OH)(s) + 3(NH₄)₂SO₄(aq)
Some magnesium will also be precipitated as magnesium hydroxide, albeit to a lesser extent, by the following formulas:
//tends primarily toward the right
MgSO₄(aq) + 2NH₃(aq) + 3H₂O(l) <-> Mg(OH)₂(s) + (NH₄)₂SO₄(aq)
MgCrO₄(aq) + 2NH₃(aq) + 3H₂O(l) <-> Mg(OH)₂(s) + (NH₄)₂CrO₄(aq)
|
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Online
Mood: interested
|
|
Yes, I also think that you made dichromate. The pictures show a nice orange color, and the fact that you get a blue solution on addition of peroxide
to the acidic solution is very strong evidence for formation of dichromate.
I am quite sure, however, that the reactions you give do not occur. Iron in the stainless steel is oxidized at the anode. The anode takes away
electrons and the cathode produces electrons.
At the anode there are many different compounds, from which electrons can be taken. Some possible reactions are:
H2O - e --> "H2O(+)" --> H(+) + OH (formation of acid and production of a radical)
The OH-radical is very reactive and immediately reacts further in many different ways. Some may combine to water and oxygen, some may be further
oxidized by the anode by taking away another electron:
OH - e --> H(+) + O, where an oxygen radical is formed, which can combine with another radical to form O2, but which also reacts with nearly
everything else.
Another reaction at the anode will be Fe - 3e --> Fe(3+) (oxidation of iron to ferric ions)
Especially all the radicals formed in the reaction mix can lead to all kinds of interesting compounds. They may result in stepwise oxidation of
Cr-atoms to CrO3, which appears as chromate or dichromate in the solution.
It is surprising to see that the chromium metal does not seem to be oxidized directly: Cr - 3e --> Cr(3+)
Apparently this does not occur, otherwise you would see a dark green color or a dark greyish color.
Another thing may be that this does occur, but that the Cr(3+) reacts with oxygen or hydroxyl radicals to something with higher oxidation state than
+3. It may even be that the anode's electron-withdrawing power is so much that chromium is oxidized all the way to "Cr(6+)", in combination with water
molecules to form chromate/dichromate.
This kind of reactions can be really complicated and many electrochemical reactions still are not (fully) understood.
Your experiment shows an interesting observation, maybe not from a practical synthetic point of view, but certainly from an academic point of view.
One very interesting thing is that insanely strong oxidizing power can be obtained by means of electrolysis. A voltage of 4 volts leads to stronger
oxidizing power than even the strongest oxidizers like F2, O2(+), XeO4 and XeF2. The redox potential for the strongest known oxidizers is somewhere
between 2 volts and 3 volts. The problem with applying that strong oxidizing power in a useful way is that the anode material itself also can be
oxidized and that is quickly is destroyed.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by SnailsAttack  |
I'm not sure how it forms, but I'm positive that there's substantial amount of soluble chromate. Turns orange in the presence of an acid, turns blue
and fizzes when I add peroxide. |
Apparently the 11 % of Cr gets electrolytically oxidised to chromate/dichromate.
That would be an interesting tidbit but really now you need to extricate the chromate from rest of the mess, for all this to have potential use.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by woelen  |
Another thing may be that this does occur, but that the Cr(3+) reacts with oxygen or hydroxyl radicals to something with higher oxidation state than
+3. It may even be that the anode's electron-withdrawing power is so much that chromium is oxidized all the way to "Cr(6+)", in combination with water
molecules to form chromate/dichromate.
This kind of reactions can be really complicated and many electrochemical reactions still are not (fully) understood.
|
And then we haven't discussed overpotentials yet:
https://chem.libretexts.org/Courses/CSU_San_Bernardino/CHEM_2100%3A_General_Chemistry_I_(Mink)/16%3A_Electrochemistry/16.07%3A_Electrolysis#:~:text=Ov
erpotential%20is%20the%20difference%20between,occur%20in%20the%20electrolytic%20cell.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 163
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Alright. Could that manifest as an electrochemical route to iron oxyhydroxide? It's what I suspect the precipitate to be made of.
Proposed reaction is as follows:
// Anode (positive electrode) reaction (redox occurs)
2Fe(s) + 4H₂O(l) -> 2FeO(OH)(s) + 3H₂(g)
Quote: Originally posted by woelen  | | ... may result in stepwise oxidation of Cr-atoms to CrO3, which appears as chromate or dichromate in the solution ... it may even be that the anode's
electron-withdrawing power is so much that chromium is oxidized all the way to "Cr(6+)", in combination with water molecules to form
chromate/dichromate. |
What do you think of these proposed reactions:
// Anode (positive electrode) reaction (redox occurs)
Cr(s) + 4H₂O(l) -> H₂CrO₄(aq) + 3H₂(g)
Followed by:
// Cathode (negative electrode) reaction (reduction occurs)
Mg²⁺(aq) + H₂CrO₄(l) -> MgCrO₄(aq) + H₂(g)
Quote: Originally posted by woelen  | | Your experiment shows an interesting observation, maybe not from a practical synthetic point of view, but certainly from an academic point of view.
|
| Quote: | | That would be an interesting tidbit but really now you need to extricate the chromate from rest of the mess, for all this to have potential use.
|
Separation by addition of ammonia solution seems to work. See my second post in this thread.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Online
Mood: interested
|
|
At the anode you certainly don't get hydrogen. Also, at the anode, electrons are withdrawn from reagents, present on it or nearby. I do not see any
electron withdrawing in your equations.
Iron oxidation almost certainly runs like Fe - 3e --> Fe(3+), where 3 electrons are taken from the iron of the anode. This indeed can be the basis
of the reaction with formation of ferric oxide/hydroxide.
At the cathode, electrons are 'pushed' onto reagents, the most common reaction at the cathode is the following:
H2O + e --> "H2O(-)" --> OH(-) + H (radical, in the past this was sometimes called nascent hydrogen).
Two of those hydrogen radicals can combine to gaseous H2 and the OH(-) ions make the liquid alkaline.
If any acid is present in the liquid, then at the cathode the mostly occurring reaction is the following:
H(+) + e --> H, followed by 2 H --> H2.
With electrolysis reactions, the number of electrons, taken away at the anode is exactly balanced by electrons, pushed onto reactants at the cathode,
so that in total, there is no charge buildup in the electrolysis cell. Charge moves through the liquid (positive ions move towards the cathode and
negative ions move towards the anode).
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Quote: Originally posted by blogfast25  |
That would be an interesting tidbit but really now you need to extricate the chromate from rest of the mess, for all this to have potential use.
|
Copper (II) chromate is insoluble (< 2 wt%), while the sulfate is soluble. So the addition of CuSO4 should give a ppt of CuCrO4, or the more common
2CuCrO4*3Cu(OH)2. Apparently this chromate dissolves in acid, though this may occur concomitantly with reduction of Cr6+. Ref attached.
Attachment: kalbus1969.pdf (2.5MB)
This file has been downloaded 115 times
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by clearly_not_atara  | Quote: Originally posted by blogfast25  |
That would be an interesting tidbit but really now you need to extricate the chromate from rest of the mess, for all this to have potential use.
|
Copper (II) chromate is insoluble (< 2 wt%), while the sulfate is soluble. So the addition of CuSO4 should give a ppt of CuCrO4, or the more common
2CuCrO4*3Cu(OH)2. Apparently this chromate dissolves in acid, though this may occur concomitantly with reduction of Cr6+. Ref attached.
|
Nice reference!
It's worth trying, seeing as chromates ('hex Cr') are now increasingly being restricted.
Starting from pure Cr (instead of SS) this couild be aviable route to Cr+6 (but there is another much simpler route)
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 163
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by woelen  | | ... at the anode, electrons are withdrawn from reagents, present on it or nearby. I do not see any electron withdrawing in your equations. ... with
electrolysis reactions, the number of electrons, taken away at the anode is exactly balanced by electrons, |
I usually try to ignore the electrons since they remain balanced across the reagents... but you're right, I can't ignore that when depicting redox
reactions within the reactor.
Do these proposed reactions look better?
| Quote: |
// Cathode (negative electrode) reaction (reduction occurs)
2H₂O(l) + 2e⁻(aq) -> H₂(g) + 2OH⁻(aq) //electrons borrowed from iron in the anode
Followed by:
// Anode (positive electrode) reaction (oxidation occurs)
Fe(s) + 3OH⁻(l) -> FeO(OH)(s) + H₂O(l) + 3e⁻(aq) //electrons returned to iron in the anode
|
and
| Quote: |
// Cathode (negative electrode) reaction (reduction occurs)
2H₂O(l) + 2e⁻(aq) -> H₂(g) + 2OH⁻(aq) //electrons borrowed from chromium in the anode
Followed by:
// Anode (positive electrode) reaction (oxidation occurs)
Cr(s) + 8OH⁻(l) -> CrO₄²⁻(aq) + 4H₂O(l) + 6e⁻(aq) //electrons returned to chromium in the anode |
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 163
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Testing has shown that the brown gunge formed at the cathode is not iron oxyhydroxide, it's some sort of iron chromate compound, perhaps iron(III)
chromate.

Gravimetric study is underway.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Online
Mood: interested
|
|
I do not think that these reaction equations, you mentioned in your post from 18-12-2022 are the ones what really occur. Also, electrons are not
returned to metals at the anode.
As a starter, think of the anode as a sink of electrons, which takes away electrons from its immediate surroundings and the cathode as a source of
electrons, which pushes electrons into its immediate surroundings.
At/near the anode, electrons are taken away. There are different candidates from which electrons can be taken:
- water molecules near the anode
- negative ions near the anode
- the material from the anode itself
The ease with which electrons can be taken determines where they are (mostly) taken from. E.g. if you have a copper anode in a solution of NaCl, then
around the anode, electrons can be taken from Cl(-) ions, from water, and from copper atoms of the anode. Taking away electrons from the copper metal
of the anode is by far the easiest in this case, and hence, the copper anode erodes. No chlorine is formed at the anode, the anode dissolves:
Cu - 2e --> Cu(2+)
If the anode is made of platinum, then it is much more difficult to take electrons from the anode material. In that case, it is easiest to take
electrons from the chloride ions, and it is slightly more difficult to take electrons from water molecules. So, both those reactions occur
simultaneously, but mostly chloride ions are stripped:
main reaction: Cl(-) - e --> Cl
side reaction: H2O - e --> "H2O(+)" --> OH + H(+)
The first reaction leads to formation of Cl2, by combination of two Cl-atoms. The second reaction mainly leads to formation of oxygen by decomposition
of the OH-radicals to oxygen and water, but there also will be traces of H2O2 and some hydroxyl radicals also may react with chlorine radicals to make
hypochloric acid. The gas mix, collected at the anode is mainly chlorine, but with a non-neglictible amount of oxygen mixed in.
At the cathode a similar reasoning can be used. At that place, electrons are pushed onto the molecules or ions, which easiest accept electrons. In the
case of a solution of NaCl, there are ions Na(+), water molecules and cathode material which can accept electrons. The cathode (for the usual metals)
cannot easily accept electrons, because that would lead to negative metal ions, which are not common at all. The sodium ions also do not easily
accept electrons. What remains are the water molecules and this reaction occurs:
H2O + e --> "H2O(-)" --> OH(-) + H
Two H-radicals then combine to H2. For some cathode metals, it also may happen that the hydrogen radicals react with the metal to form a metal
hydride. This can be a secondary side-reaction. With copper, however, this does not occur.
If instead one uses a dilute solution of CuCl2 instead of NaCl, then at the cathode, there are Cu(2+) ions instead of Na(+) ions, which can accept
electrons. Cu(2+) ions accept electrons more easily than water molecules and hence, in that situation, copper metal is formed at the cathode and no
(or hardly any) hydrogen is formed.
---------------------------------------------------
In your case, list all of the materials around the anode, which can donate electrons and order them with the ease at which they can donate. Do the
same for the cathode area.
Write down all primary reactions near the anode, which you can imagine by taking away electrons. Do the same for the cathode area for reactions, in
which electrons are accepted.
Your situation is much more complicated than the NaCl-case, I gave above in order to demonstrate my way of reasoning. In your case, there are many
secondary reactions, which lead to formation of dichromate or chromate. I think that the formation of your brown ferric chromate most likely is a
combination of a primary reaction and a secondary reaction:
primary: Fe - 3e --> Fe(3+)
secondary: Fe(3+) goes into solution and reactions with previously formed chromate to form ferric chromate, or more likely some basic ferric chromate.
The brwon material you have may have a composition close to Fe(OH)CrO4.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 163
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
I've encountered 2 complications so far relevant to my post on December 21st.
1. The pyrolyzed iron oxyhydroxide is hygroscopic and can pull 5% its weight in water from the air within 15 minutes of cooling to below ~150°C. Mass
measurements must be done quickly.
2. The contradance displacement reaction between ammonium chromate and zinc acetate (to yield ammonium acetate and insoluble zinc chromate) doesn't
proceed below a certain concentration. In practice, the solution must be evaporated before any zinc chromate precipitates. This behavior is also
exhibited by a solution of magnesium sulphate and sodium bicarbonate. Not sure what causes it.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 163
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Test is on hold indefinitely. Precipitating chromate with zinc salts is too unreliable, and I have to start all over. Not sure when I'll get around to
it. Bummer.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by SnailsAttack  | | The contradance displacement reaction between ammonium chromate and zinc acetate (to yield ammonium acetate and insoluble zinc chromate) doesn't
proceed below a certain concentration. In practice, the solution must be evaporated before any zinc chromate precipitates. This behavior is also
exhibited by a solution of magnesium sulphate and sodium bicarbonate. Not sure what causes it. |
Are you aware of the concept of Solubility Product (KS)?
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 163
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
That's just the solubility, yeah? I prefer working with g/L. Magnesium carbonate is insoluble enough that it should precipitate from solutions of ~10%
magnesium sulphate and ~10% sodium bicarbonate immediately. And uh... it just doesn't, last I checked. The solution has to be evaporated before the
metathesis reaction proceeds.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Not exactly. You can find the relation between KS and S here:
http://www.sciencemadness.org/talk/viewthread.php?tid=65303#...
In the case of ZnCrO4,
KS= [Zn2+] x [CrO42-] =1 x 10-8 (*)
which is not as low as you might think.
Bear also in mind that the solubility of chromate is pH dependent.
(*) Approximate value
[Edited on 6-1-2023 by blogfast25]
|
|
|